BioByte 091: quantum biology, a foundation model for drug repurposing, functional genomic variant interpretation, and new mechanisms in brain metabolism
Welcome to Decoding Bio’s BioByte: each week our writing collective highlight notable news—from the latest scientific papers to the latest funding rounds—and everything in between. All in one place.
What we read
Bessemer’s Healthcare AI Thesis [Morgan Cheatham, Bessemer and Decoding Bio]
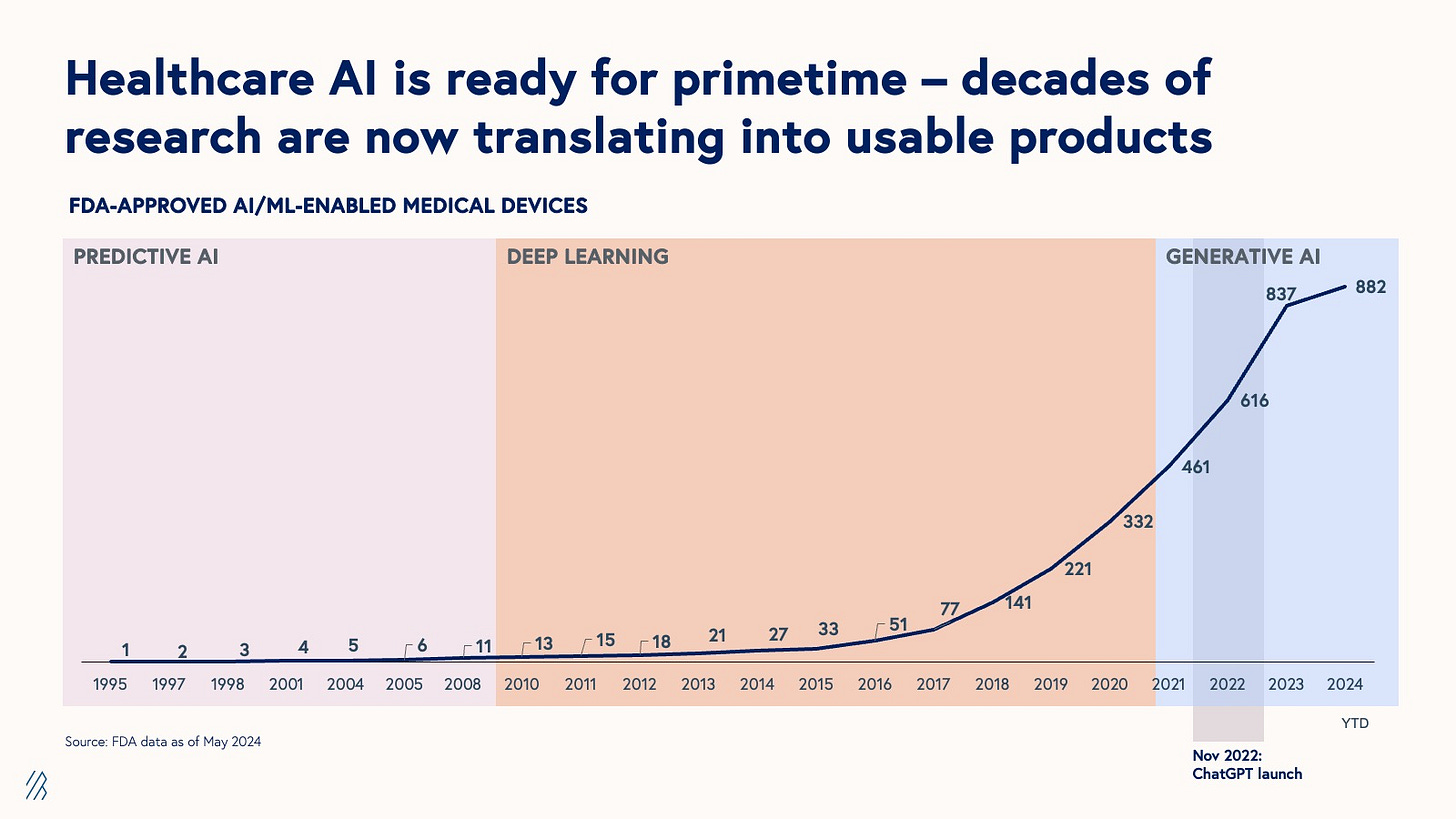
Bessemer Venture Partners has released a comprehensive roadmap on AI in healthcare, highlighting that the industry is at a tipping point with unprecedented demand and innovation. Healthcare produces 30% of the world's data, now largely digitized, enabling AI to redefine our understanding of health from molecular to population levels. Despite challenges like complex regulations and unique market forces, AI research is rapidly translating into tangible products and services.
Bessemer emphasizes that successful healthcare AI companies will focus on modality-business model-market fit, leverage multimodal AI, and develop vertical-specific infrastructure. They're targeting investments in core technology themes like interactive systems and specialty foundation models across $1 billion+ healthcare verticals. The most impactful companies will be those that fundamentally reimagine healthcare processes, not just automate existing ones.
About quantum biology [The Guy Foundation]
The Guy Foundation, a charity founded in 2018 to facilitate exploration into quantum effects in biology and the role it could play in advancing medicine, has a fascinating summary on how quantum mechanics affects biology. Quantum mechanics, which describes the behavior of matter and energy at the smallest scales, reveals that even large molecules can display both particle-like and wave-like characteristics. Some examples:
The problem of decoherence: It was previously believed that the quantum properties of molecules would be disrupted by the ‘wet and warm’ biological environments, limiting their relevance to living systems. Recent research suggests that the warmth of biological systems might not hinder, but rather amplify certain quantum effects, potentially playing a role in the evolution of life itself.
Quantum tunneling: The transfer of energy in living systems may be enhanced by quantum tunneling, allowing particles to bypass energy barriers that would otherwise be insurmountable.
Coherent energy and charge transfer: Quantum coherence allows biological systems to explore multiple energy pathways simultaneously, potentially increasing the efficiency of processes like photosynthesis.
Entanglement: Quantum entanglement (aka “spooky action at a distance), the connection between particles, might underlie phenomena like avian navigation and could even play a role in brain function and consciousness.
The revolution in high-throughput proteomics and AI [Topol, Science, September 2024]
Recent advancements in high-throughput proteomics now allow measurement of thousands of plasma proteins from tiny blood samples, providing rich data that can be integrated with genetic and lifestyle information to transform our understanding of human health and aging. Studies using these techniques have identified organ-specific aging clocks for 11 different organs, predicted risk for over 50 common and rare diseases, and assessed overall mortality risk. Proteomic research has revealed that aging is not a linear process, with protein expression peaking at different ages throughout life. By integrating proteomic data with other health information and employing AI analytics, researchers are enhancing our ability to predict disease risk and understand aging processes. While current proteomic assessments are expensive, the technology shows promise for future routine medical care through the development of targeted, lower-cost protein panels. These developments in proteomics and AI are opening new frontiers in personalized medicine and health prediction, potentially revolutionizing how we approach disease prevention and treatment.
The prion principle and Alzheimer’s disease [Walker et al., Science, September 2024]
Understanding the mechanism of a disease is critical for developing therapeutics. Obtaining this understanding at early stages of the disease is particularly important, as this is when interventions are often most effective. Significant steps have recently been made to advance the understanding of the origins of Alzeheimers, which appears to be surprisingly similar to the onset of prion diseases.
Prion diseases—such as Creutzfeldt-Jakob disease or Gerstmann-Sträussler-Scheinker syndrome—are characterized by misfolded prion protein aggregates. In Alzheimer’s, amyloid-β and tau proteins undergo a similar process as the disease progresses. Being increasingly understood as the first defining feature of Alzheimer’s, the formation of the amyloid-β aggregates is particularly of interest.
In one experiment seeking to understand the role of amyloid-β, researchers found that pituitary gland exposure from cadavers with small amyloid-β aggregates (seeds) led to increased rates of larger amyloid-β clots years down the line. Another study was recently published on children who received transfers of human growth hormone that was again extracted from cadavers. Three decades after the transfer, a significant subset of these patients expressed clinical characteristics of Alzheimer’s. Causation is difficult to prove, but the results of the studies not only warn of the dangers of human-derived biologics but also seem to indicate that the formation of amyloid-β aggregates via propagation of seeds is important to the onset of Alzheimer’s.
With a greater understanding of the possible mechanism of the beginnings of Alzheimer’s and its similarity to prion diseases, scientists can now focus on understanding this mechanism of misfolding, propagation, and aggregation to focus therapeutic strategies for these diseases.
Oligodendroglial fatty acid metabolism as a central nervous system energy reserve [Asadollahi et al., Nature Neuro, Sep 2024]
Oligodendrocytes, the cells responsible for creating myelin in the central nervous system, play a crucial role in supporting neuronal health and function. A paper published in Nature Neuroscience this month investigated the relationship between oligodendrocytes, energy metabolism, and axon function in low-glucose environments. Using isolated optic nerves from mice, the study found that oligodendrocytes can provide energy reserves for axons during periods of glucose deprivation through fatty acid oxidation. This process helps maintain axon function and prevents cell death in low-glucose conditions.
The experiments also showed that when glucose uptake by oligodendrocytes is disrupted, myelin loss occurs even in the absence of behavioral deficits. This suggests that in diseases associated with reduced glucose metabolism, such as Alzheimer's, the imbalance between myelin production and degradation may contribute to progressive myelin loss. These findings highlight the importance of oligodendrocytes in providing energy support to axons and maintaining myelin integrity, particularly in conditions of metabolic stress or during aging. This research provides new insights into the complex relationship between brain metabolism, myelin maintenance, and neuronal function, which could have implications for understanding and treating neurodegenerative diseases.
Benchmarking algorithms for single-cell multiomics prediction and integration [Hu et al., Nature Methods, Sept 2024]
Single-cell multi-omics technologies are advancing our understanding of cellular biology, but integrating diverse data types like RNA expression, protein abundance, and chromatin accessibility remains complex. A recent study published in Nature Methods assessed 14 algorithms for predicting protein levels and chromatin accessibility from scRNA-seq data, as well as 18 algorithms for integrating single-cell multi-omics data. The analysis utilized 47 different datasets to evaluate performance in predicting and combining these diverse biological layers.
The results showed that totalVI and scArches stood out as the most accurate for predicting protein levels, while LS_Lab was the top choice for predicting chromatin accessibility. In terms of integrating different types of data, Seurat, MOJITOO, and scAI performed best for vertical integration, while totalVI and UINMF excelled in tasks that involved merging multi-batch RNA and chromatin data.
By comparing key performance metrics such as Pearson correlation, AUROC, and RMSE, the study provided detailed insights into how each algorithm functions in both single-dataset and cross-dataset contexts. The research highlights the importance of choosing the right tool based on specific needs and demonstrates the challenges of multi-omics integration. This benchmarking framework offers researchers valuable guidance for selecting optimal algorithms to explore complex biological processes.
Deciphering the impact of genomic variation on function [IGVF Consortium]
The Impact of Genomic Variation on Function (IGVF) Consortium, launched by the National Human Genome Research Institute in 2021, aims to systematically understand how genomic variations affect genome function and shape phenotypes. This ambitious project brings together over 120 laboratories in a coordinated effort to unlock the vast potential of human genomics for understanding biology and improving health. The IGVF employs a "map-perturb-predict" framework, integrating single-cell mapping, systematic genomic perturbations, and predictive modeling to create a comprehensive catalog of genome function and variant effects.
The consortium focuses on understanding effects on gene regulation, protein function, and molecular networks, with applications in clinical variant interpretation, uncovering disease mechanisms, and ensuring findings are applicable across diverse populations. To support the scientific community, the IGVF will create the IGVF Catalog for data exploration, the IGVF Data Portal for accessing datasets, and open-source computational methods and pipelines. This groundbreaking project welcomes collaborations and promises to accelerate our understanding of genomic variation's role in human biology and disease, potentially revolutionizing genetic diagnosis and therapeutic development.
A foundation model for clinician-centered drug repurposing [Huang et al., Nature Medicine, September 2024]
Researchers have developed TxGNN, a groundbreaking artificial intelligence model for drug repurposing that can identify potential new uses for existing drugs across 17,080 diseases. TxGNN uses a graph neural network and metric learning to analyze a vast medical knowledge graph, predicting both indications and contraindications for drugs. Unlike previous models, TxGNN can make "zero-shot" predictions for diseases with limited treatment options or no existing drugs.
The model outperforms eight existing methods, improving prediction accuracy for indications by 49.2% and contraindications by 35.1% in zero-shot evaluations. TxGNN includes an Explainer module that provides transparent insights into its predictions through multi-hop interpretable paths. Human evaluation showed that TxGNN's predictions and explanations perform well on multiple aspects beyond just accuracy. Many of TxGNN's new predictions align with off-label prescriptions made by clinicians in a large healthcare system. The researchers believe TxGNN could accelerate drug repurposing efforts, particularly for rare and complex diseases with few treatment options.
Notable Happenings
Generate Biomedicines partners for $65M upfront with Novartis
Founded by Flagship Pioneering, Generate Biomedicines has quickly established itself as a frontrunner in using AI to revolutionize protein therapeutics. The company has raised over $600 million to date, enabling it to build state-of-the-art cryo-electron microscopy (cryo-EM) laboratories in Andover, Massachusetts. These facilities allow Generate to produce proprietary cryo-EM data, which feeds into their AI models like Chroma, released in late 2023.
One of Generate’s notable advancements is its anti-TSLP, which is nearing a critical data readout. The success of this antibody could validate the effectiveness of their AI-driven platform and open doors to new therapeutic avenues. The upfront payment is on the higher end compared to other AI-pharma partnerships, such as Gilead’s $35 million to Genesis and Novartis’s $38 million to Isomorphic. However, it’s difficult to interpret fully given the uncertainties around the number of targets and the specifics of the collaboration.
For Novartis, such deals are valuable when dealing with challenging targets where it provides biological insights but lacks the ability to drug them on its own. These partnerships can help unlock new targets, diversify the pipeline, and enhance Novartis’s understanding and presence in AI-driven drug discovery.
Read an interview with Generate’s CTO and Decoding Bio
George Church’s ‘GC Therapeutics’ raises $75M in funding
A new start-up from the Church lab has developed a ‘non-biased’ combinatorial screen to more efficiently differentiate induced pluripotent stem cells (iPSCs) into specialized cell types, enabling their broader application across regenerative medicine. The method also produces differentiated human cells in a single-step process within four days, a significant improvement compared to current iPSC differentiation methods, which often take weeks or even months. GC was founded by Parastoo Khoshakhlagh and Alex Ng, investors include Cormorant Asset Management and a16z.
Other happenings:
ARCH Venture Partners raises $3B to invest in the next-generation of life science companies
Flagship unveils Mirai Bio with the goal of creating nanoparticle-guided genetic medicines
858 Therapeutics raises $50M Series B for phase I solid tumor asset
BioAge to raise $198M in IPO - the company is developing a medicine to boost the effects of GLP-1 agonists, however, how much of this IPO size is due to market hype or true innovation?
Resync Bio launches to automate data and operations in drug discovery
Field Trip
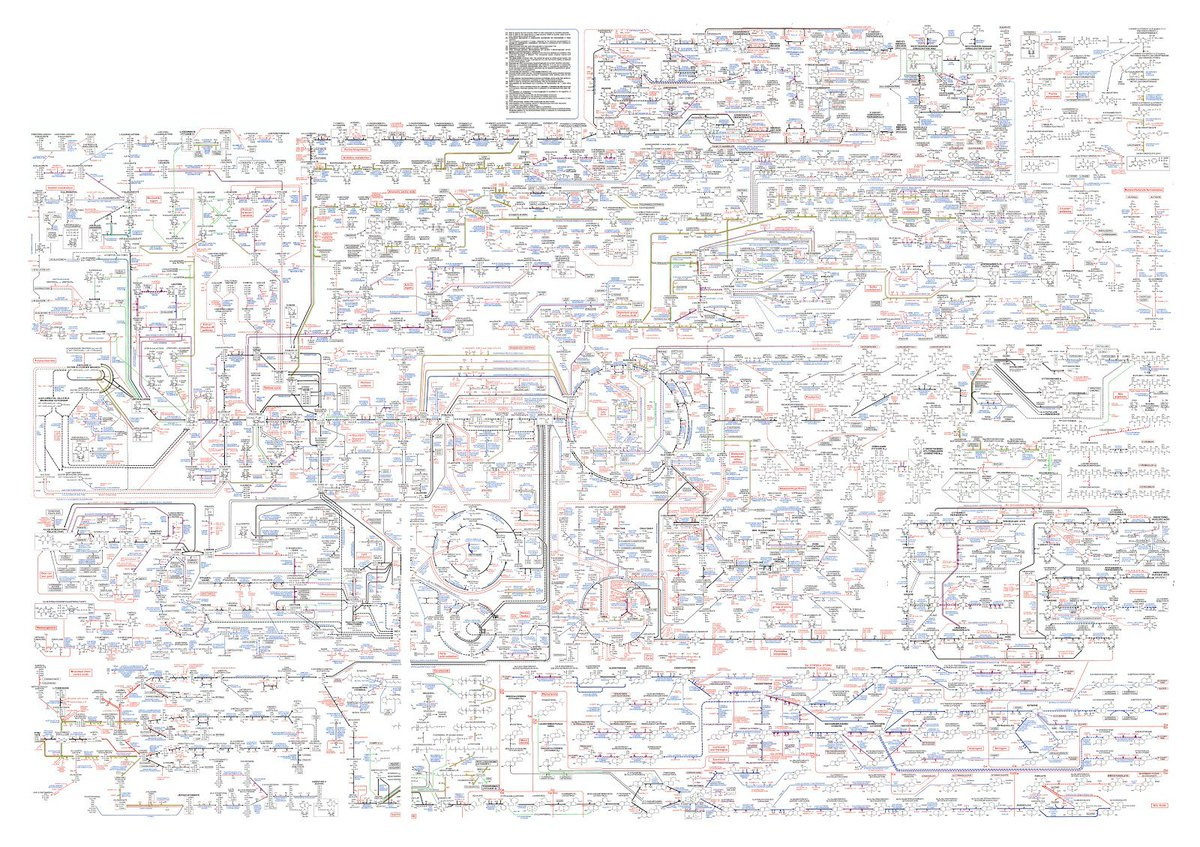
A simplified view of metabolism: