BioByte 080: "Bust to Boom" in biotech, gene editing in the lungs, advancements in colonic organoids, increasing access to pediatric CGT
Welcome to Decoding Bio’s BioByte: each week our writing collective highlight notable news—from the latest scientific papers to the latest funding rounds—and everything in between. All in one place.
Summer’s here! We’re busy cooking up a number of exciting events, collaborations, and content here at Decoding after re-launching our platform.
If you haven’t yet, check out our new website.
Interested in collaborating? Drop us a line!
What we read
Evolution Of The Biotech IPO Markets From Busted To Booming [LifeSciVC, 2020]
This 4 year old blog post from LifeSciVC describes some of the historical up- and downswings in the biotech IPO market driven by technology hype and macro environment.
In 2004, the IPO markets were just opening after the bubbles in tech and genomics in 2001. Alnylam and Momenta were going public, but those IPOs were painful: both priced around 50% below the mid-point of the expected price range. For most of the 2001-2011 period, most IPOs were below range and valuations extremely constrained.
After 2011, several large biotechs drove significant outperformance of the NASDAQ Biotech Index, drawing more investors into the sector increasing the depth of the capital markets, and coupled with the increased rate of innovation it drove up interest and valuations in the space.
But this doesn’t explain why IPO pricing became more efficient. This occurred due to the JOBS act in 2012, which allowed for confidential S1 filing and to test-the-waters to understand pricing of an IPO before publicly disclosing that a company intends to go public. When this post was written, IPO pricing was at an all time high. Of course, as interest rates increased post-pandemic, alpha-seeking investors retreated to less risky assets.
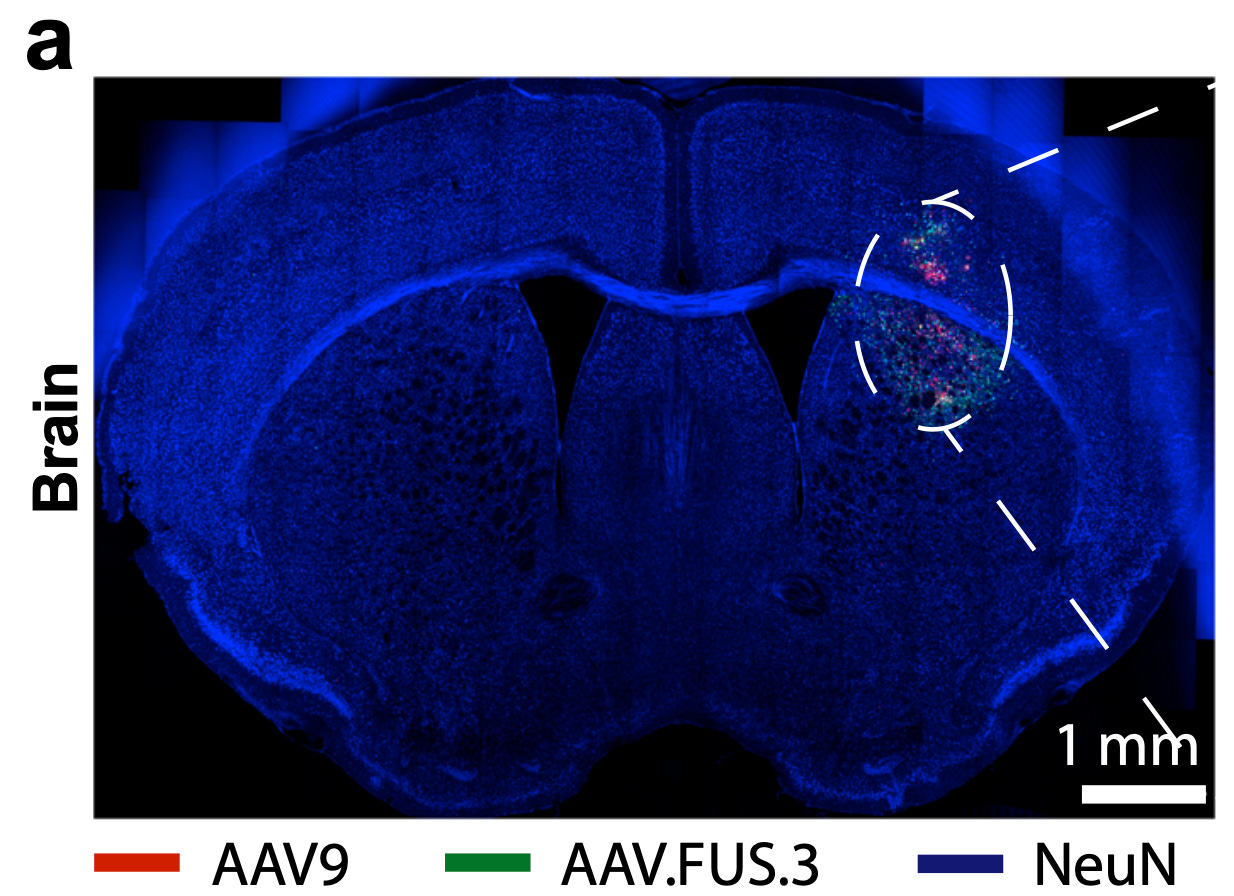
Engineering viral vectors for acoustically targeted gene delivery [Li et al., Nature Communications, June 2024]
Delivering gene therapies to specific regions of the brain remains a challenge. Current methods are either invasive (e.g. injections in the spinal cord, called intrathecal delivery) or lack spatial precision. Focused ultrasound blood-brain barrier opening (FUS-BBBO) is a noninvasive technique that enables targeted delivery of gene therapy vectors, such as AAVs, to specific brain regions by transiently and reversibly opening the BBB. However, when used with natural AAV serotypes, FUS-BBBO has limited transduction efficiency and can result in undesirable transduction of peripheral organs. The authors hypothesized that engineering AAV vectors specifically designed for FUS-BBBO delivery could enhance the efficacy and specificity of this approach.
In this study, high-throughput in vivo selection was used to engineer new AAV vectors optimized for local neuronal transduction at the site of FUS-BBBO. A library of AAV capsid variants was screened in mice, selecting for efficient and specific transduction in the FUS-targeted brain hemisphere. The resulting engineered vectors, named AAV.FUS candidates, were then characterized in detail. The top candidate, AAV.FUS.3, demonstrated a more than ten-fold improvement in brain-targeting specificity compared to the commonly used AAV9 in two mouse strains (figure below). AAV.FUS.3 exhibited enhanced transduction efficiency in the FUS-targeted brain regions, reduced off-target transduction in peripheral organs like the liver, and increased neuronal tropism.
China has become a scientific superpower [The Economist, June 2024]
The Economist recently published an article highlighting China's transformation in the scientific landscape. The article asserts that China has evolved from a reputation of "copy-cat" and "lower quality" science to outpacing many Western countries in both the quantity and innovation of high-quality research.
Source: The Economist
The Economist also published a figure visualizing what sub-fields of science that China particularly excels in. It is interesting to note its lead in applied sciences presumably due to engineering innovations needed to keep up with China’s rapid economic growth. For example, China leads in electric vehicle batteries as well as research into perovskite solar panels.
Two key reasons are outlined in the article as contributing to this rise. Firstly, an increase in spending of 16-fold since 2000, in line with its economic growth. Secondly, incentive schemes to attract top Chinese talent back into the country, such as the “Youth Thousand Talents” that offered researchers $150k to set up their own labs.
Although China’s output has growth, the share that is conducted with international collaborators has remained the same and increasingly political tensions may jeopardize this further.
Gene editing flows to the lungs [Bulcaen and Carlon, Science, 2024]
The delivery of gene editing modalities to the lungs has been historically challenging, due largely to protective mechanisms, including a robust epithelial barrier, mucus sweeping, and phagocytic cell populations. Indeed, over 27+ clinical trials for cystic fibrosis (CF) have failed as they were unable to overcome the airway epithelium, highlighting yet again how delivery is one of the most challenging aspects to gene therapy.
The authors highlight recent work from Sun et al. 2024, who were able to intravenously deliver lung-targeted lipid nanoparticles containing CRISPR components in a mouse model of cystic fibrosis. Cystic fibrosis is a prototypical autosomal recessive disorder in which mutations derange the chloride transporter CF transmembrane conductance regulator (CFTR) gene, leading to severe mucus buildup and propensity for infection. Although inhalational therapeutic approaches have been attempted several times in the past, these have failed due to ciliary beating, dense mucus layers, and the scarcity of available relevant receptors. Sun et al. attempted to bypass this entirely, by approaching the lung from the basolateral side. This comes with its own challenges – these LNPs have to then migrate past the endothelium of capillaries, stromal cells, and the relevant supporting structures before reaching the airway basal cells, which are the long-lasting, multipotent stem cells in the lung.
Sun and team demonstrate the ability to reach several lung cell types, including the basal cells; however, the mechanism of how LNPs migrate through blood vessels to the airway epithelium remains poorly understood. However, LNPs are able to bind to soluble proteins in the blood (including vitronectin, a key component for pulmonary entry), which allows them facile entry into the lung. Results are exceptional: they were able to deliver gene editing to nearly half of all lung cells, with 45 to 80% efficacy in basal cells. These LNPs loaded with CRISPR base editing components were able to restore function in the cystic fibrosis mouse model. This is exciting as work has shown that possibly 1.5 to 10% restoration of wild-type CFTR function is clinically meaningful.
It’s important to note that intravenous delivery comes with its own disadvantages: mainly, the whole body is open for targeting. The authors demonstrate CFTR editing in the pancreas, spleen, liver, and intestines; although this may be beneficial in CF as it is a multi-organ disease, there remains significant work to assess toxicity and genomic integrity across the body.
Bioengineered human colon organoids with in vivo-like cellular complexity and function [Cell Stem Cell, 2024]
Roche’s Institute of Human Biology have published a paper on their mini-colons, a hugely improved in vitro model of the human colon. The team combined concepts from typical organoids as well as dynamic fluid flow of organs-on-a-chip to create their mini-colon, using hydrogel engineering, microfabrication and human stem cell culture. The importance of truly representative organoids would be transformational for preclinical testing and maximizing further clinical success. Some of the previous challenges of colon organoids have been truly replicating the environmental milieu, spatial organization and cell type diversity. However, the team show with scRNA-seq and immunohistochemistry to have replicating cell diversity and perform simple experiments showing the utility of their models in various functions such as modeling nutrient and drug uptake.
Enhancing Pediatric Access to Cell and Gene Therapies [Mackall et al., Nature Medicine, June 2024]
Cell and gene therapies (CGTs) are transforming the treatment landscape for pediatric diseases, yet their development and commercialization face significant hurdles due to small market sizes, high manufacturing costs, regulatory challenges, and inadequate licensing practices. These obstacles often lead to a market failure where potentially life-saving treatments do not reach the children who need them. To address this, experts propose creating a new entity, the Pediatric Advanced Medicines Biotech (PAMB), which would spearhead the late-stage development and commercialization of pediatric CGTs outside the traditional biopharmaceutical model. This entity would leverage the academic ecosystem for manufacturing and work closely with regulatory bodies to ensure timely approvals and access.
Advancements in CGTs have shown impressive results in treating pediatric conditions. For example, Tisagenlecleucel (Kymriah) has significantly improved survival rates in children with refractory B-cell acute lymphoblastic leukemia, and Onasemnogene abeparvovec-xioi (Zolgensma) has shown remarkable efficacy in treating spinal muscular atrophy. Despite these successes, the high cost of development and the small patient populations for many pediatric diseases make it challenging to justify the investment under the current biopharmaceutical model.
The proposed PAMB would address these challenges by partnering with academic institutions to access innovative therapies, utilizing academic GMP facilities to reduce manufacturing costs, and adopting new regulatory approaches to expedite approvals. By focusing on pediatric-specific licensing practices, the PAMB aims to ensure that CGTs are developed and made available to children in a timely and cost-effective manner.
Notable Deals
Roche bets on Ascidian Therapeutics’ RNA exon editing technology - the duo have partnered to tackle neurological disorders. Roche will pay Ascidian $42M upfront.
Belharra Therapeutics receives $40M upfront from Sanofi for its non-covalent chemoproteomics platform in immunology.
Marea Therapeutics raises $190M to target reduce lingering cholesterol usually left behind despite treatment.
Versant incubated Santa Ana Therapeutics launches with $168M to bring three precision autoimmune therapies to clinic by 2025.
Prolific Machines raises $55M - the start-up is using light to directly control the exact level and duration of cellular processes. Applications include nutritional and therapeutic protein production, cultured meat, disease models and cell therapy.
In case you missed it
Events
AI x Bio Summit 2024 @ NYSE: Sign up here for the reception. Space is very limited!
Field Trip
Did we miss anything? Would you like to contribute to Decoding Bio by writing a guest post? Drop us a note here or chat with us on Twitter: @ameekapadia @ketanyerneni @morgancheatham @pablolubroth @patricksmalone