BioByte 118: terraforming Mars, broadly neutralizing antibodies targeting HIV, bioprinting inside the body, and AI modeled on neurons for continuous thought
Welcome to Decoding Bio’s BioByte: each week our writing collective highlight notable news—from the latest scientific papers to the latest funding rounds—and everything in between. All in one place.
We warmly invite the Decoding Bio community to join us for a reception to close out our third annual AI x Bio Summit on July 22. Enjoy an evening of wine and conversation beneath the lights of the storied New York Stock Exchange trading floor as we reflect, connect, and celebrate. It’s a chance to engage with fellow founders, researchers, and operators shaping the future of biology and technology. We can’t wait to see you there! Please register here
What we read
Blogs
The case for Mars terraforming research [Alden DeBenedictis et al., Nature Astronomy, May 2025]
Can we terraform Mars? Since 1991, its feasibility has not been systematically addressed. Ongoing developments in three areas have advanced meaningfully since then: climate engineering and modelling, extremophile biology and synthetic biology and space science. In fact, the emergence of Mars transport vehicles such as Starship will broaden the scope of missions by increasing the mass that can be launched by 100x per Mars landing with a >1000x improvement in cost to the surface.
The authors consider three thresholds in making Mars suitable for life:
Abiotic environmental engineering to heat up the planet. Temperatures should increase by 30 °C to start to melt the ice to enable agricultural and ecological succession. This would require solar sails as mirrors to collect and reflect additional light onto the planet. Enhancing the greenhouse effect via silica aerogels or aerosols is also possible.
Ecological engineering using extremophiles (such as autotrophic, aerobic primary producers), followed by algae and plants. The initial microbes will have to be engineered to withstand Mars’ five primary stressors: low pressure, oxychlorine species, low temperature, radiation, and low water activity. Some existing species can already withstand some of these stressors.
Biosphere development using complex plant life and trees, with increased oxygen content and atmospheric pressure. Oxygenation by photosynthesis to a 0.1-bar oxygen atmosphere (to allow for breathability and reduce radiation) would take millennia, so water electrolysis is also required.
Fully terraforming Mars will be a multicentury project. The authors suggest that as corporations and governments contemplate terraforming, science should play an important role. That is only possible if scientific research keeps up with space access technology.
Scientists Can Now 3D Print Tissues Directly Inside the Body—No Surgery Needed [Shelly Fan, singularityhub, May 2025]
As tissues degrade in the body, repair and implants become necessary, though they generally imply intense surgical procedures. 3D tissue printing allows for greater customization of implantable tissues, however, still requires these intensive surgeries. In order to circumvent this, researchers at Caltech have introduced a new methodology that enables 3D printing of tissues within the body.
Established bioprinting methods construct layers one-by-one, with each layer being solidified by light. This technique has recently been improved via volumetric printing which sets the printed tissues with a highly specific, targeted blast of either light or sound. To this end, both infrared light and ultrasound have been used, though the former is limited by the light’s propensity to scatter the deeper it penetrates into the body, constraining its implant-printing capabilities to only a few millimeters beneath the skin. Ultrasound, on the other hand, can safely reach much deeper—up to eight inches—into musculature and organs. Furthermore, Zhang et al. (2023) devised a chemical mixture dubbed “sono-ink” due to its inherent property of reactivity to sonic waves. Still, this ultrasound-activated sono-ink was found to have significant sensitivity to mechanical stress, heat, and other bodily disruptions, all of which led to reduction in print quality and resolution. Heat in particular proved problematic as it led to elevated biocompatibility risks and premature hardening of the printed structures.
The Caltech group’s approach, termed deep tissue in vivo sound printing (DISP), is characterized by an upgraded version of Zhang et al.’s sono-ink and ameliorates the dilemmas caused by heat. The new method proved greatly effective in improving control over the print and successful penetration into musculature to produce precise structures. Their ink consists of three parts: a compound which forms the bioprint when activated, the activator, and additional chemicals to assist with monitoring the progress of the bioprint. The ingenuity of the ink lies in the activator, which is held in fatty bubbles until released with ultrasonic stimulus, catalyzing the assembly of the print. This was tested successfully on both thick cuts of pork and chicken, as well as in mice and rabbits, i.e. in demonstrating its usefulness in applications like extending the lifetime of bladder cancer drugs around the tumor site, overcoming a significant shortcoming of bladder cancer treatments today.
The ink is also highly customizable and can even include components that allow for the creation of physiological sensors on tissues of interest. Despite the potential for this ink, it is not without its own limitations. Different tissues each interact with sound in unique ways, hampering generalizability and potentially posing profound impacts to the curing process. The prints have also yet to be tested on organs that move, such as the heart. The authors suggest that this might present an opportunity for AI integration, as such algorithms may offer increased capabilities toward deciphering variables and guiding the printing process. Nevertheless, especially considering these new developments, bioprinting is an undeniably exciting area to watch and holds significant promise of treatment for patients across a myriad of indications.
Papers
Precise targeting of HIV broadly neutralizing antibody precursors in humans [Caniels et al., Science, May 2025]
HIV has proven unusually hard to vaccinate against because the virus cloaks itself in a dense sugar shield and mutates rapidly. A small subset of people eventually make “broadly neutralizing antibodies” (bnAbs) that thread through this shield and block many HIV strains, but the naïve B‑cell precursors that can mature into bnAbs are extremely scarce and normally ignored by the immune system. “Germline‑targeting” vaccines aim to solve that by giving the immune system an Env protein that is specially engineered to fit those rare precursors and coax them onto the right evolutionary path. Caniels et al. tested the first such trimer, BG505 SOSIP v4.1‑GT1.1, in humans—an essential proof‑of‑principle for a staged HIV vaccine that would later steer those primed cells toward full breadth.
In a randomized, double‑blind phase‑1 study, three intramuscular doses of GT1.1 adjuvanted with AS01B were safe and well‑tolerated, producing only mild‑to‑moderate local reactogenicity. All participants developed robust binding‑IgG responses to the trimer, clearing the first safety and immunogenicity hurdles for advancing to booster immunogens. High‑dose recipients showed notably stronger responses than the low‑dose arm.
Most critically, the vaccine accomplished its molecular mission: it expanded VRC01‑class precursors that recognize the CD4 receptor‑binding site. In the high‑dose group roughly 1 in 2 500 class‑switched memory‑B cells now carried VRC01‑class receptors—a ∼10‑fold enrichment over low dose—and 30 % of all CD4bs‑specific clones belonged to that class. Those lineages had already accumulated ≥5 % somatic hypermutation and bore hallmark adaptations such as glycine indels in CDRL1 that accommodate the key N276 glycan. Expressed monoclonals mirrored this progress: >80 % bound shaping trimers with N276, one‑third bound fully glycosylated BG505 with sub‑nanomolar affinity, and a subset neutralized tier‑2 pseudoviruses. Cryo‑EM confirmed true VRC01‑like angles of approach, with some antibodies already slipping past the intact N276 glycan.
SYNOPSIS: A three‑shot priming with the GT1.1 Env trimer safely “awakens” the rare B‑cells needed for broadly neutralizing antibodies and nudges them part‑way down the mutational road toward potency, marking a pivotal first step on the multi‑stage path to an effective HIV vaccine.
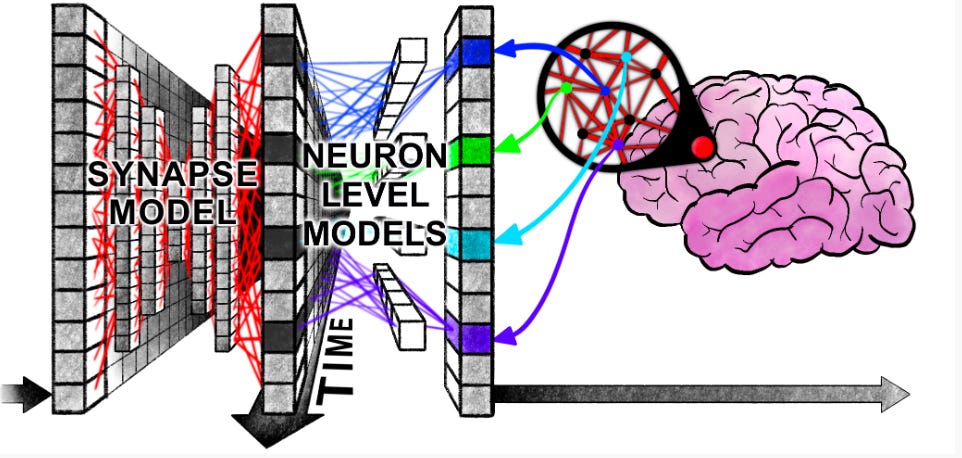
Continuous Thought Machines [Darlow et al., arXiv, May 2025]
Artificial neural networks (ANNs) are loosely inspired by the human brain but omit much of the rich, time-based behavior that makes biological learning so flexible. Real neurons communicate with precisely timed spikes, adjust connection strengths through spike timing and frequency, and generate oscillations to coordinate large networks of cells. These dynamics allow brains to learn new skills from very few examples, adapt to novel situations, and generalise across tasks. In contrast, conventional networks collapse each neuron’s activity into a single number per step and propagate those values in lockstep, which favors computational efficiency but sacrifices many of the adaptive benefits of timing. The Sakana AI team’s Continuous Thought Machines (CTM) paper introduces a neural architecture that uses timing as a core element of processing. There are a few key steps which enable this. Firstly, the model has an internal “thinking” loop where the model runs for a fixed number of discrete “ticks” that are separate from the input steps. At each tick, it refines its latent state so that harder cases get more ticks of compute. Secondly, each neuron carries its own tiny recurrent network that updates its activation based on that neuron’s recent pre-activations over the last few ticks, giving each unit its own little memory and rich temporal dynamics. Thirdly, the model builds a synchronisation matrix (essentially pairwise, exponentially-weighted dot-products of each neuron’s activation history) and samples from it to form (a) the output logits and (b) the attention/query vectors. In other words, timing relationships between neurons are the features the network reasons over. To assess its versatility and the power of its dynamics the CTM was tested on a broad suite of tasks using the same core architecture. In image classification it matched or exceeded baselines on ImageNet-1K, often allowing early stopping without sacrificing accuracy. In sequential reasoning it learned to solve large maze-navigation problems and “draw” its own paths, and in algorithmic settings it mastered sorting real numbers and computing parity with near-perfect generalization.
Notable deals
GlycoEra has raised a $130M series B to advance treatments for autoimmune disorders. The funding round was led by Novo Holdings and included new investors Catalio Capital, LifeArc Ventures and QIA alongside existing investors Sofinnova, 5AM, Roche Ventures and BMS. GlycoEra is developing degraders to target IgG4 autoantibodies in indications including pemphigus vulgaris.
AusperBio has raised an additional $50M as part of its Series B round. The funds will be used to advance AusperBio’s oligonucleotide therapies in hepatitis B. Investors in the round included Qiming Venture Partners, CDH Investments, Genesis Capital, YuanBio Venture Capital, HanKang Capital and Sherpa Capital.
Grin Therapeutics has raised a $140M series D. The round was led by Angelini Pharma and Blackstone Life Sciences. Grin Therapeutics is developing therapeutics for pediatric neurodevelopmental disorders. Grin Therapeutics and Angelini Pharma have also entered a collaboration for the development of radiprodil.
Biogen maps out $1B biobucks deal with RNAi-focused City Therapeutics. Biogen and City Therapeutics are entering into a research agreement to work on a target mediating CNS diseases using City’s RNAi engineering technology.
Syndeio Biosciences has launched with $90M raised to date. Investors included Catalio Capital Management, Innoviva, Tenmile, Luson Bioventures, Palo Santo, AbbVie and Eli Lilly. Syndeio is developing therapies to restore and enhance synaptic health in central nervous system disorders. Syndeio’s lead asset, zelquistinel, is currently in Phase 2 trials for major depressive disorder.
In case you missed it
Gilgamesh links psychedelic to 90% remission rate in midphase depression trial
What we liked on socials channels
Field Trip
Did we miss anything? Would you like to contribute to Decoding Bio by writing a guest post? Drop us a note here or chat with us on Twitter: @decodingbio.