BioByte 117: biomanufacturing in space, predicting clinical trial outcomes, programmable bridge RNAs, and continuous evolution of CRISPR-associated transposases
Welcome to Decoding Bio’s BioByte: each week our writing collective highlight notable news—from the latest scientific papers to the latest funding rounds—and everything in between. All in one place.
We warmly invite the Decoding Bio community to join us for a reception to close out our third annual AI x Bio Summit on July 22. Enjoy an evening of wine and conversation beneath the lights of the storied New York Stock Exchange trading floor as we reflect, connect, and celebrate. It’s a chance to engage with fellow founders, researchers, and operators shaping the future of biology and technology. We can’t wait to see you there! Please register here
What we read
Blogs
Boosters and Biologics: Is Space-Based Biomanufacturing Real? [Cody Tranbarger, Life Sci VC, May 2025]
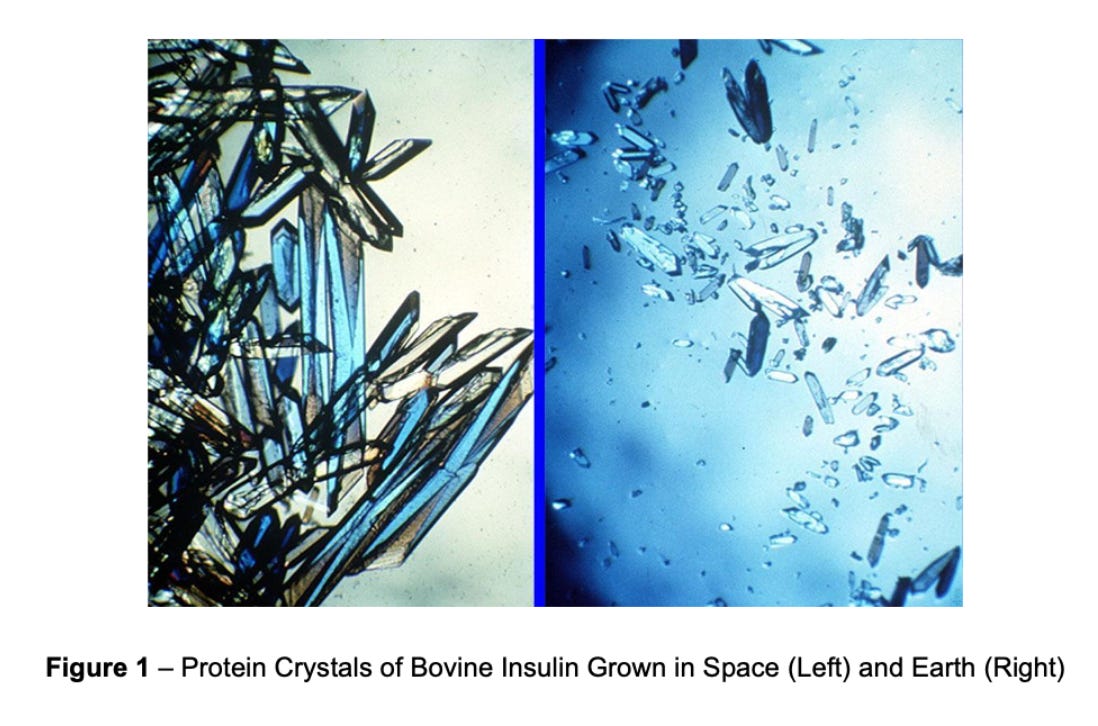
Over the past 30 years, biopharma has been conducting microgravity research in space. In recent years, several companies have appeared with the vision of manufacturing drugs in space. For instance, Varda Space launched a bioprocessing capsule into Low-Earth Orbit, creating a unique crystal of the HIV drug ritonavir and returning the material to Earth intact.
The lowest hanging fruit for commercialization, the author describes, is the use of microgravity-based crystallization to improve properties of drugs. Microgravity enables the growth of larger, more uniform protein crystals.
Why is this valuable? This is demonstrated by Merck’s Keytruda’s case study. In a study on the ISS, Merck generated a Keytruda suspension with higher particle homogeneity and lower viscosity relative to those on earth. This showed superior compatibility with subcutaneous delivery. Convenience is increasingly important in the pharmaceutical industry; new “hard-to-copy” formulations could unlock meaningful competitive differentiation.
There are three business models appearing in the space-based life science sector:
CDMO: provides space-based manufacturing e.g. Varda
CRO: provides research-as-a-service e.g. Yuri Gravity, Redwire, SpacePharma
Real Estate / Infrastructure (REIT): developers of commercial space stations and satellites that will host life science R&D e.g. Axiom Space
The author argues that “IP-enhanced” CDMOs that provide unique value, such as cell lines (Lonza) or formulations (Halozyme), can escape the fee-for-service paradigm of CROs and capture better economics via milestones and royalties. This model of lower volume, greater value is more compatible with the restrictions of current space-based manufacturing and the most economically viable model to venture-scale returns.
There are some challenges ahead: operational cost of bioreactors in space are hard to predict, achieving the same scale as terrestrial manufacturing has not been achieved (and already difficult on Earth to transition to larger bioreactor volumes) and there is an uncertain regulatory path. Importantly, as with many manufacturing technologies in biopharma, it suffers from a chicken and egg problem: adoption is required to prove reliability and reproducibility, but in order for companies to adopt it they need to see the same qualities.
With fine-tuned AI models, OpenAI, Babylon aim to predict clinical trial successes [Conor Hale, Fierce Biotech, May 2025]
Drug failures in clinical trials often spell disaster for small biotechs and big pharma alike. Estimates put costs to the industry at around $45 billion annually, often resulting in failed deals and acquisitions as well as major layoffs. Babylon Biosciences and OpenAI are seeking a solution. The two have been working together to train large language models to predict potential points of failure in the drug development process via reinforcement fine-tuning and a programmable numeric grading system. Using biomedical data from Sleuth Insights—including 430 clinical trials spanning a variety of indications—researchers on this project are adapting a version of OpenAI’s o3-mini model to drive the desired improvements in predictive capabilities, leveraging the power of LLMs to synthesize sparse, multimodal data (e.g. mechanism of action and preclinical results) to draw conclusions.
“That binary result of clinical trials—sink or swim—offers a unique opportunity to gauge improvements as AI models receive more training” - Babylon CEO, Sacha Schermerhorn
When presented with a blinded set of studies, the team has already found success increasing the prediction accuracy up from an area-under-the-curve of 0.65 to 0.84. These remarkable strides allow for far greater competitiveness for smaller biotechs like Babylon, enabling them to not only move faster, but also do so with greater confidence. Babylon seeks to use the advantage their tech will impart to license potential molecules for development with a focus on Alzheimer’s and neurological conditions.
While initially hesitant himself about AI, Schermerhorn now asserts that “one day every company will have its own personalized AI model.” Still, their model has not as of yet been phase-differentiated, and like all LLMs, is only as good as the data fed to it. By its nature, historical data used for training does not consist of very diverse patient populations, nor does it comprise the telling failures that are often omitted from reporting. The latter can lead to an overly optimistic model, while the former will only demonstrate improvement with better trial recruitment. Nevertheless, the model has already shown propensity for generating instrumental, non-obvious insights that can assist significantly with resource allocation and deriving future directions. With the potential to save exponential amounts of time and money, these efforts will likely serve to revolutionize the drug development industry.
Papers
Megabase-scale human genome rearrangement with programmable bridge recombinases [Perry et al., bioRxiv, May 2025]
Scientists at the Arc Institute have engineered programmable bridge recombinases to relevant levels of genome editing in human cells, enabling scarless insertion, excision, and inversion of DNA at unprecedented genomic scales.
Previously, the team mined metagenomes to discover classes of recombinases, and discovered through extensive biochemical characterization that they are programmable through their own set of guide RNAs, called bridge RNAs. This RNA-based programmability is what made CRISPR-Cas9 such a powerful technology - with it, scientists can clearly define what part of the genome they want to target, and the programmability has led to technologies like CRISPR-based pool screening. Bridge recombinases extend two significant capabilities that CRISPR-Cas9 doesn’t. First, bridge RNAs are programmable for both the target and the donor DNA. Second, the mechanism of recombination doesn’t create double stranded breaks (DSBs), which lead to problematic genomic errors such as insertions and deletions.
Building on an initial screen of 72 IS110 orthologs in human cells, the authors identified IS622 as a robustly active recombinase. Through structure-guided engineering of its bRNA—modulating stem lengths and introducing stabilizing point mutations—and a complementary deep mutational scan of the recombinase protein itself, they created an enhanced two-component system. These optimizations yielded up to a 3.8-fold boost in bRNA activity, an additional ~1.5-fold gain from protein mutations, and, in combination, achieved insertion efficiencies of up to 20% with genome-wide specificities as high as 82%.
Beyond simple insertions, the platform supports megabase-scale rearrangements, enabling precise inversions of up to 0.93 Mb and excisions of up to 0.13 Mb with no apparent distance dependency. To demonstrate this therapeutic potential, they used the IS622 to perform scarless excision of the BCL11A enhancer—a key target for sickle cell anemia—and removal of expanded GAA repeats implicated in Friedreich’s ataxia. These proof-of-concept edits underscore the promise of bridge recombinases for both functional genomics and gene therapy.
Programmable gene insertion in human cells with a laboratory-evolved CRISPR-associated transposase [Witte et al., Science, May 2025]
Researchers have harnessed laboratory-driven evolution to engineer a powerful new CRISPR-associated transposase system called evoCAST, dramatically enhancing the precision and scope of programmable gene insertion into human cells.
The evoCAST system leverages phage-assisted continuous evolution (PACE) to evolve Type I-F CRISPR-associated transposases, significantly improving their ability to seamlessly integrate large DNA sequences without causing double-strand breaks or genomic scarring. Just like the bridge recombinases in Perry et al. 2025, this lack of genomic scarring is a key advantage over CRISPR-Cas9. Similar to CRISPR-Cas9, evoCAST relies on RNA-guided targeting, allowing researchers to easily direct integration events to specific genomic loci. However, evoCAST surpasses traditional CRISPR by enabling direct insertion of multi-kilobase cargoes with single-base precision and remarkably low off-target integration.
Through extensive characterization, evoCAST demonstrated robust integration efficiencies of 10–30% across diverse genomic sites in both HEK293T and primary fibroblast cells, consistently producing highly specific edits without undesired insertions or deletions. The technology's clinical promise was underscored by its capability to insert large therapeutic payloads at safe-harbor sites and into loci relevant to genetic diseases.
By combining evolutionary biology with advanced CRISPR engineering, evoCAST establishes a versatile and highly precise platform for therapeutic gene insertion. This transformative capability positions evoCAST as an essential advancement in genome-editing technologies, offering profound implications for genetic research and personalized medicine.
Robin: A multi-agent system for automating scientific discovery [Ghareeb et al., arXiv, May 2025]
FutureHouse are back with another release, named by another bird: Robin. Robin is a system that orchestrates its literature-search agents (Crow and Falcon) with the data-analysis agent (Finch) to autonomously propose scientific hypotheses for therapeutic discovery, as well as designing a plan to test this. The team performed a proof-of-concept on dry age-related macular degeneration (dAMD). The Robin system identified ripasudil, a previously approved ROCK inhibitor as a novel candidate for AMD.
To identify a potential novel treatment for dAMD, Robin reviewed over 150 papers to hypothesise that enhancing retinal pigment epithelium phagocytosis could treat the eye condition. Robin then used Crow to propose several experimental assays that could be used to test the efficacy of a candidate set of molecules in treating dAMD via its proposed mechanism. After the phagocytosis enhancement assay was chosen, researchers at the Future House lab executed the assays and fed the results back into the system for data analysis and to inform Robin’s next selection of potential therapeutic candidates. After several cycles, Robin landed on ripasudil (a clinically approved ROCK inhibitor) as a novel therapeutic hit. This workflow showcases a powerful proof of concept for end-to-end AI driven discovery, yet its reliance on careful prompt engineering, curated literature access and human in-the-loop validation reminds us that Robin is an exciting step toward automated science rather than a replacement for skilled researchers.
Figure: Architecture and workflow of the Robin system
Notable deals
23andMe acquired by Regeneron for $256M. After filing for bankruptcy, 23andMe has been acquired by Regeneron. This builds on Regeneron’s long-time commitment to using genetic databases to understand disease biology. Regeneron also plans to continue all consumer genome services run by 23andMe.
Juvenescence has raised a $76M Series B round led by M42 alongside existing investors. This is the first tranche of their Series B round with the second close expected to occur in Q3. The funding will be used to continue Juvenescence’s goal of developing a pipeline of AI-enabled therapeutics to extend healthy lifespan and advance the treatment of life-threatening diseases.
ReproNovo has received $65M in a funding let by Jeito Capital. AXA IM Alts and M Ventures co-led the financing with Ysios Capital and ALSA Ventures also contributing. The company aims to use these funds to advance RPN-001 and RPN-002. RPN-001 is an oral therapy for male infertility and RPN-002 is a first-in-class compound to manage adenomyosis.
CellCentric has raised a $120M series C round. The round was co-led by RA Capital and new investor Forbion alongside other investors including Avego Bioscience Capital, BrightEdge and the venture capital and impact investment arm of the American Cancer Society. The funds will be used to advance clinical studies of inobrodib by initiating a Phase II/III study and support development activities of a Phase III program.
Orionis Biosciences announces strategic partnership with Genentech. Genentech has extended its collaboration with Orionis to develop molecule glue medicines for cancer. Orionis will receive an upfront payment of $105M and receive future milestone payments whose total could exceed $2B.
Pfizer pays 3SBio $1.25B for PD-1xVEGF bispecific. This is $1.25B upfront with up to $4.8B in milestones for SSGJ-707. SSGJ-707 is going to start phase 3 clinical trials in China, and is undergoing four phase 2 trials in various cancer types, also in China. This comes after Merck and BioNTech themselves made $588M and $800M purchases of PD-1/L1xVEGF bispecifics. This move also adds to the increasing trend of Big Pharma looking to China for M&A.
In case you missed it
World’s first personalized CRISPR therapy given to baby with genetic disease
A base-editing therapy was developed for a child with mutations from both parents for carbamoyl phosphate synthetase 1, which leads to ammonia buildup. Only about half of babies with this CPS-1 deficiency survive long enough to receive a transplant, which is the standard of care.
What we listened to
What we liked on socials channels
Field Trip
Did we miss anything? Would you like to contribute to Decoding Bio by writing a guest post? Drop us a note here or chat with us on Twitter: @decodingbio.

















hey Decoding!
Given your interest in Biotech manufacturing, you might enjoy my recent biotech piece on eXoZymes Inc. They’ve just commercially launched a cell-free enzyme-based biomanufacturing platform that converts biofeedstocks into targeted chemical products.
Plus they just announced their first subsidary which synthesises N-trans-caffeoyltyramine (NCT) to treat MASLD/MASH. Very very interesting compound that has immense potential
https://www.slack-capital.com/p/exozymes-research-report