BioByte 113: solving the epigenome, psychopathological computations in LLMs, a novel protein circuit for mutated Ras, and desmosome mutations in skin cutaneous melanoma
Welcome to Decoding Bio’s BioByte: each week our writing collective highlight notable news—from the latest scientific papers to the latest funding rounds—and everything in between. All in one place.
🚨 [Last day to apply - applications close TONIGHT] We're looking to expand the Decoding Bio team with Product Managers to transition Your Missing Guide to Breakthrough Biotech into a media empire. If you're interested in envisioning and implementing the future of Decoding Bio, fill out the quick form linked to the button below! We’ve already received many applications and are excited to be reading every single one in the coming weeks. 🚨
What we read
Blogs
Cellular alchemy: How solving the epigenome will let us create every healthy cell in the body [Zack Chiang, Cellular Alchemy, April 2025]
Since before the time of the alchemist, the pursuit of extending life has captivated a multitude of minds. These early attempts to prolong life with mysterious concoctions were ill-conceived, but with the last few decades of advances in biology, understanding how to lengthen the human lifespan is closer to reality than ever.
In 1962, John Gurdon made the first significant step in longevity research when he demonstrated that the nucleus of a frog embryo could be replaced with one from a mature skin cell and produce a healthy frog, proving that the nucleus of all cells contains the entire genome. Forty years later, Shinya Yamanaka discovered a set of four transcription factors that could be used to convert mature skin cells into induced pluripotent stem cells (iPSCs), reverting all signs of aging in the process. The allure of this “cellular alchemy” where scientists can reprogram cells to alter their function and age has spurred not only significant academic research, but also investment from Silicon Valley, with companies like Altos Labs receiving $500 million from Jeff Bezos to work on rejuvenation. However, the author argues that the current techniques used to understand cellular reprogramming are insufficient to grasp the complex and nuanced interplay of the fundamental mechanisms that underlie it. They believe solving the epigenome to be the solution.
In the context of this article, the epigenome is considered to be the central processing unit of the cell, the piece of hardware that consolidates a myriad of signals and translates them into phenotypic outputs. The author believes that solving it means the creation of a generalizable framework that can be used to create healthy cells of any type in the human body. In order to solve the epigenome, there needs to be a way to study it, which is where the systematic manipulate-measure-model approach—based on Ed Boyden’s approach to solving the brain—becomes relevant.
In seeking to manipulate the epigenome, a balance between precision and scale must be struck. CRISPR technologies are highly specific, yet difficult to scale. Epigenetic drugs have the opposite problem. Transcription factors strike the right balance, but hijacking them for targeted applications could prove to be challenging due to their complexity. With their three domains, it is likely that a combination of top-down and bottom-up approaches will need to be taken to successfully manipulate the epigenome at scale.
For measurement, the author proposes a multi-modal technique called expansion in situ genome sequencing (ExIGS). The method combines expansion microscopy and DNA sequencing to connect nuclear phenotypes and epigenetic states. There are still limitations to the technique, especially with respect to temporal dynamics, but the spatial resolution it affords is a significant advancement in understanding the epigenome. The author also believes ExIGS can be akin to what cryo-EM was for AlphaFold, enabling the creation of models that can accurately predict cellular outcomes in a given environment.
The topic of longevity and cellular reprogramming is polarizing, not only from a scientific standpoint, but also an ethical one. There are significant concerns over how to distribute these technologies equitably such that everyone can have access to longer, healthier lives. Encouraging these conversations while investing in this research because of the potential value it affords will help bring about an equitable era of health abundance.
Papers
Emergence of psychopathological computations in large language models [Yong Lee et al., aRxiv, April 2025]
Can AI systems implement computations of psychopathology? The possibility of psychopathological computations in LLMs raises concerns as they could disrupt human-machine interactions, introduce safety risks or be exploited by bad actors to introduce harmful dysfunctions.
In order to explore this question, we require an account of psychopathology applicable to computational entities without biological embodiment or qualia (subjective experiences). The authors define psychopathology as “a state of being trapped within self-sustaining symptoms due to their causal cyclicity” and its analogous computation interpretation as “temporal patterns of unit values, driven by a structural pattern of rules”.
To test their hypothesis, the team used an LLM to generate synthetic datasets containing sentences expressing symptoms in human psychopathology with thought and intensity labels. These are used as both interventions (exogenous variables) and to understand the representational state of the model. In order to measure and intervene in the computational units (i.e. the symptoms) in LLM, the authors used Sentence-level Supervised Sparse AutoEncoder (S3AE), which uses supervised learning signals to make a targeted identification and intervention of the thought-level representational states in LLMs.
In short, the paper hypothesizes and corroborates that network-theoretic computations of psychopathology have already emerged in LLMs. As the authors put it “LLMs can be trapped within the spread and self-sustenance of dysfunctional and problematic representational states, driven by their dynamic and cyclic [units]”. A possible explanation is that during training, LLMs capture patterns analogous to human thought processes, including those that are dysfunctional. This does not imply that these systems experience distress as seen in human pathologies.
Engineered protein circuits for cancer therapy [Lu et al., bioRxiv, April 2025]
Ras mutations underpin 1 in 4 cancers. While traditional small molecule inhibitors have emerged to target these oncogenic mutations, they often face limitations due to the development of acquired resistance. This resistance can arise through various mechanisms, such as the downregulation of Ras or the emergence of new Ras mutations, ultimately limiting the long-term effectiveness of these pharmacological approaches. How can we treat oncogenic Ras mutations without allowing cancers to develop resistance?
To address the challenge of resistance, a team of researchers from the labs of Michael Elowitz, Hao Zhu, and Daniel Siegwart developed a novel protein circuit-based therapeutic strategy. They engineered a modular sensor-amplifier-effector circuit designed to recognize the specific structure of dominant mutant Ras proteins. This recognition directly triggers a controlled cell death pathway, utilizing either apoptosis or pyroptosis. Key components of their circuit include a monobody, initially developed in Shohei Koide’s lab, which serves as a highly selective sensor for mutant Ras, and an inventive new amplification method they devised to boost the signal from the protease-based activation mechanism. The complete circuits were effectively delivered to cells using mRNA encapsulated in lipid nanoparticles (LNPs).
The researchers demonstrated the therapeutic potential of their protein circuits in vivo by delivering them via mRNA-containing LNPs to liver tumors in mice. In these studies, circuits incorporating the pyroptosis effector successfully treated the aggressive liver cancer. Crucially, in head-to-head in vitro comparisons against the FDA-approved KRASG12C inhibitor Sotorasib and the pan-Ras inhibitor RMC-7977, the protein circuit exhibited superior performance, achieving more complete killing of mutant KRAS cells while sparing wild-type cells. Furthermore, RNA-seq analysis revealed that the protein circuit minimally affected global gene expression patterns in healthy cells, unlike the small molecule inhibitors which caused more widespread transcriptomic changes, suggesting the circuit may be a less disruptive therapeutic option.
This invention is highly significant as it establishes a potent, specific, and programmable approach to cancer therapy by directly linking disease-specific signals to therapeutic outcomes. The demonstrated advantages in overcoming resistance compared to existing targeted drugs highlight its potential to improve treatment durability. The modular nature of the circuits allows for potential adaptation to sense various disease markers and trigger different cellular responses - theoretically this platform can extend beyond Ras-driven cancers and even beyond oncology.
Desmosome mutations impact the tumour microenvironment to promote melanoma proliferation [Baron et al., Nature Genetics, April 2025]
In patients with skin cutaneous melanoma (SCM), the tumour microenvironment can play a key role in melanocyte transformation and cancer progression. Desmosomes are membrane proteins complexes that act as cell-cell adhesion molecules and have previously been shown to play a role in tumorigenesis.
In this study, the authors analysed The Cancer Genome Atlas (TCGA) to establish the prevalence of mutations in the genes encoding the desmosome complex. They observed that mutations in desmosome genes were present in over 70% of cases. The most frequently mutated desmosome gene was desmoplakin (DSP). The authors then established that these mutations result in a decrease in desmosome gene expression. Analysis of gene expression patterns revealed that this decrease in expression occurs in keratinocytes rather than in primary melanoma cells. Keratinocytes engineered with reduced expression of a desmosome subunit resulted in increased proliferation of nearby melanoma cells. The authors propose that mutations in desmosome genes may result in a skin microenvironment that favours malignant transformation.
This study is important because it establishes that non-tumour cells can carry mutations that play an important role in cancer progression. This supports the idea that tumours arise because of effects from multiple cell types rather than a single clonal population.
Notable deals
Repertoire Immune Medicines forms a partnership with Genentech for $35M upfront to help develop treatments for an undisclosed autoimmune disease. Repetoire’s ‘Decode’ platform deciphers the immune synapse by identifying antigens with MEDi™, mapping T cell responses with CIPHER™, and pinpointing T cell receptor specificities using MCR™. An integrated machine learning tool, NEPTUNE, then synthesizes these data to guide the development of precise immunotherapies. Repertoire will use its platform and know-how to lead early discovery work in the collaboration.
Alis Biosciences, a new British investment fund, aims to unlock tens of billions of dollars trapped in the balance sheets of nearly 300 struggling public biotechs with depressed stock prices and sizable cash reserves. The fund proposes innovative strategies to recycle this capital by delisting companies, placing assets in special vehicles, and providing flexible returns to shareholders while supporting drug development, an approach that has garnered strong investor and analyst support.
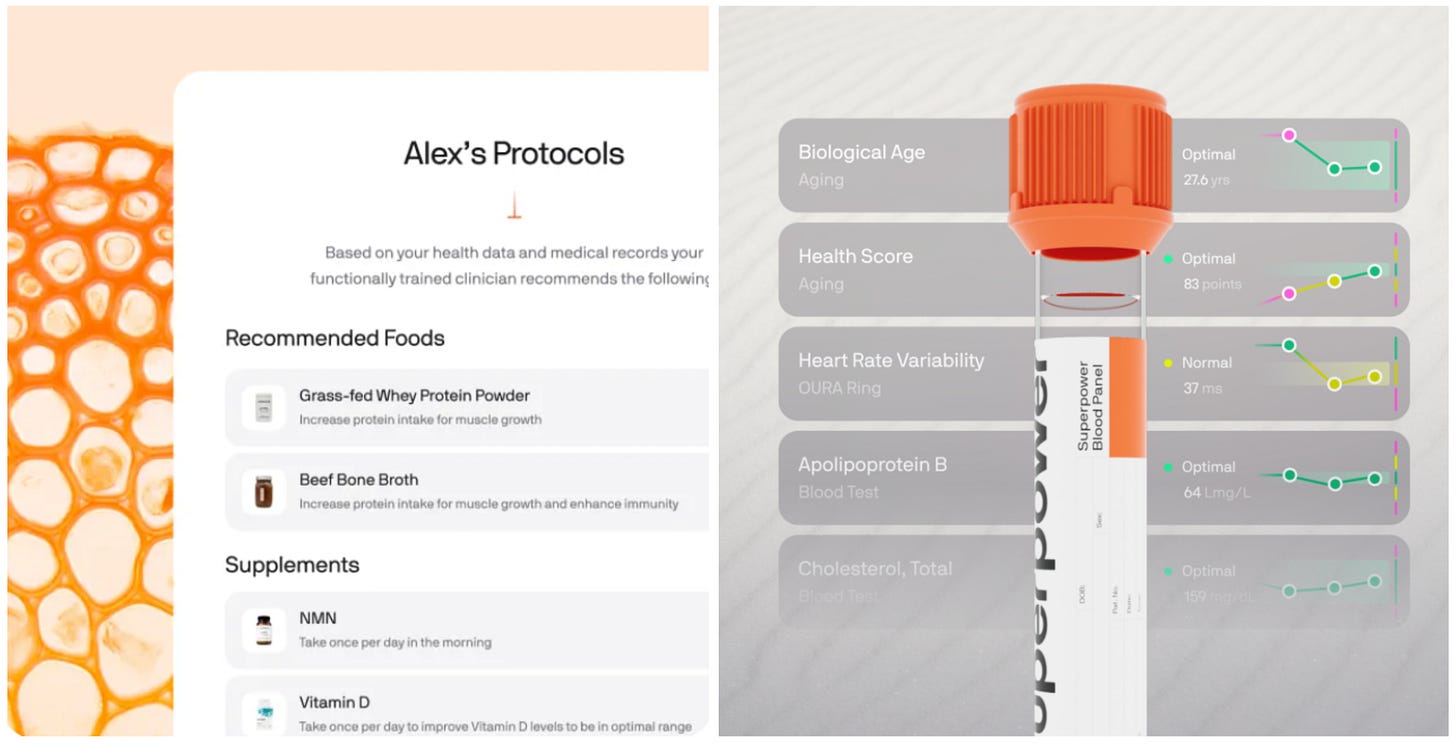
SF-based Superpower has launched with a $30M backing to develop a consumer-focused preventative healthcare platform. For a yearly subscription fee of $499, users receive access to two comprehensive lab tests covering over 100 biomarkers, which are analyzed by Superpower’s platform to generate 17 distinct health scores. The system integrates data from popular wearables like Oura and Whoop while offering personalized health protocols, a marketplace for supplements, and access to health support. In essence, the platform empowers users to own and track their health data, enabling proactive discussions with physicians when needed.
Grove Biopharma closed a $30 million Series A financing round to advance its Bionic Biologics platform, a novel technology that creates fully synthetic, cell permeable drug candidates to target challenging intracellular protein protein interactions. Initially focused on oncology and neurodegenerative diseases with a lead program in castrate-resistant prostate cancer, the technology represents a new paradigm in drug development for previously intractable targets.
Roche announced a $50B investment in U.S. manufacturing and R&D over the next five years, creating 1,000 jobs. The move follows similar U.S. investment plans including $23B from Novartis, $55B from Johnson & Johnson, and $27B from Eli Lilly. It comes amid newly imposed 31% tariffs on Swiss imports, prompting a broader shift by European pharma to localize operations.
What we listened to
What we liked on socials channels
Field Trip
Did we miss anything? Would you like to contribute to Decoding Bio by writing a guest post? Drop us a note here.