BioByte 109: new gene targets for bipolar disorder, mechanisms of the meningeal lymphatic-microglia axis, and an abundance of new AI models for drug discovery
Welcome to Decoding Bio’s BioByte: each week our writing collective highlight notable news—from the latest scientific papers to the latest funding rounds—and everything in between. All in one place.
What we read
Blogs
Neo-1: Decode and Design the Structure of Life (VantAI, March 2025)
VantAI introduces their first foundation model, Neo-1, which unifies structure prediction and molecule generation in a single framework. VantAI’s aim has been to make protein interactions programmable. By reprogramming proteins already present in the cell using small molecules, they can direct these proteins to engage with aberrant targets. This process gives rise to what are known as “Neoproteins”, which can perform new functions in the cell and provide a basis for treating a wide array of diseases.
The key challenge in this approach is that when a small molecule binds to a target protein, it changes the protein’s structure in order to react with the disease protein. This new structure cannot be predicted from the protein sequence alone. Traditional models work in separate steps, first predicting a structure from a sequence and then designing a molecule to interact with that structure. Neo-1, however, handles these tasks simultaneously by working in a latent space where sequence and structure information are integrated. This method reduces error accumulation and offers better control, making it possible to generate novel molecules that induce the desired structural changes in proteins.
In essence, Neo-1 provides an integrated solution that paves the way for more precise and efficient drug discovery. It is a step forward in creating programmable biology where both the structure of the protein and the small molecule that modifies it are designed in tandem to achieve therapeutic outcomes.
Papers
TxAgent: An AI Agent for Therapeutic Reasoning Across a Universe of Tools [Gao et al., bioRxiv, March 2025]
TxAgent is an AI agent that leverages multi-step reasoning and real-time biomedical knowledge retrieval across tools to analyze drug interactions, contraindications, and patient-specific treatment strategies.
To prescribe the appropriate drug, one needs to evaluate multiple factors, including “patient-specific characteristics, comorbidities, drug interactions, contraindications, current clinical guidelines, drug mechanisms of action, and the underlying biology of the disease”. LLMs fail at real-time access of medical data, accuracy and reasoning over multiple variables. Tool-augmented LLMs, however, can incorporate mechanisms such as RAG to mitigate some of these issues. TxAgent overcomes all of these limitations by generating natural language responses alongside a transparent reasoning trace, describing each step of its decision-making process.
In order to execute on complex medical queries (see examples in Figure F through I), the agent uses ToolUniverse, a biomedical toolbox consolidating 211 tools spanning drug mechanisms, interaction, clinical guidelines and disease annotations (Figure C). The agent also employs ToolRag, a model which selects the most relevant tools from ToolUniverse based on query context.
Figures D and E show how TxAgent (fine-tuned Llama 3.1-8B-Instruct) outperformed GPT4o and Llama 3.1-70B-Instruct on drug reasoning tasks (DrugPC). Compared to DeepSeek-R1 (671B parameter model), TxAgent achieves 7.5% higher accuracy in open-ended queries; demonstrating specialized reasoning and tool-use capabilities outweigh model size.
More info:
TxAgent project is at https://zitniklab.hms.harvard.edu/TxAgent
TxAgent code and demos are at https://github.com/mims-harvard/TxAgent
ToolUniverse is at https://github.com/mims-harvard/ToolUniverse
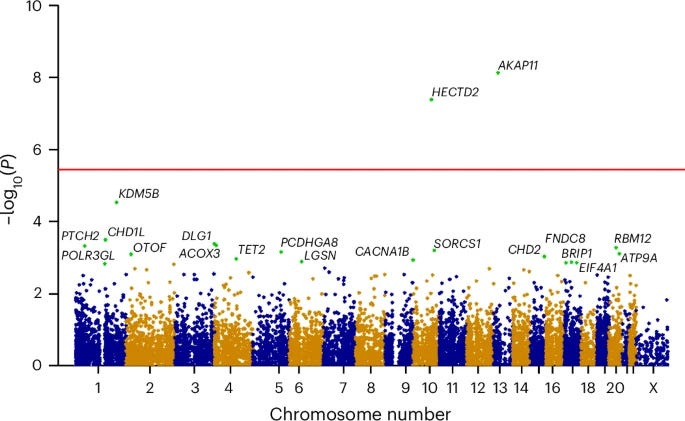
Rare loss-of-function variants in HECTD2 and AKAP11 confer risk of bipolar disorder [Thorgeirsson et al., Nature Genetics, March 2025]
There is an urgent need for better treatments for bipolar disorder. Identifying biological targets that drive the disorder is a critical step toward developing new interventions. Bipolar disorder is highly heritable but to date the number of targets identified with genetics has been limited.
In a new study from deCODE Genetics and Amgen, researchers have identified two novel genes (AKAP11 and HECTD2) whose loss-of-function is associated with bipolar disorder. The authors conducted a genome-wide LoF burden meta-analysis, combining data from both the Icelandic population and the UK Biobank to increase statistical power. AKAP11 has previously been implicated in schizophrenia, underscoring potential shared genetic architecture across psychiatric conditions. HECTD2, however, represents a novel association with no prior link to bipolar disorder. Interestingly, both gene products are known to interact with GSK3β, a protein that may be a key target of lithium, one of the few treatments currently in use for bipolar disorder.
These findings highlight AKAP11 and HECTD2 as compelling candidates for therapeutic development.
TXGemma: Efficient and Agentic LLMs for Therapeutics [Wang et al., Google DeepMind, March 2025]
This week, Google DeepMind unveiled TxGemma, a new family of open-source language models built to supercharge therapeutic development. Trained on over 7 million examples from the Therapeutics Data Commons, TxGemma is designed to predict drug properties, reason through complex biomedical questions, and power interactive agentic systems.
TxGemma includes models with 2B, 9B, and 27B parameters, fine-tuned on over 7 million datapoints across small molecules, proteins, nucleic acids, diseases, and cell lines. It’s designed to serve as a generalist model for therapeutics, outperforming specialist models in many tasks and requiring less data to fine-tune on downstream problems.
TxGemma addresses three core use cases:
1. TxGemma-Predict
Fine-tuned on the Therapeutics Data Commons (TDC), this model supports prediction tasks like binary classification (ex. does this molecule cross the blood-brain barrier?), regression (ex. predicting binding affinity), and generation (ex. synthesizing new molecules via SMILES). TxGemma-Predict outperforms the state-of-the-art generalist model on 64 of 66 tasks, and beats specialist models on 26. It also requires less fine-tuning data, making it ideal for data-scarce therapeutic problems.
2. TxGemma-Chat
TxGemma-Chat brings natural language reasoning into the drug discovery loop. It can be used to justify predictions with molecular rationale, engage in conversational exploration (ex. "Why might this compound be toxic?"), and serve as an educational and collaborative research assistant. Unlike prior models, TxGemma-Chat blends domain-specific training with general instruction tuning, allowing it to retain both conversational fluency and domain awareness.
3. Agentic-Tx
Powered by Gemini 2.0, this agentic system orchestrates complex workflows using a modular toolset of 18 specialized tools, including predictive engines (toxicity, binding affinity, trial success), knowledge tools (PubMed search, gene/protein databases), and molecular tools (SMILES processing, descriptor extraction). Agentic-Tx uses the ReAct framework to chain thoughts and actions—reasoning, invoking tools, and synthesizing final answers. It outperforms GPT-4o, Claude 3.5, and o3-mini on benchmarks like ChemBench and Humanity’s Last Exam, showcasing how LLMs can go beyond single-step predictions.
TXGemma is an interesting new open source model that can be used to engage with data and workflows in an individual manner. It’ll be interesting to track how it will be integrated into daily lab workflows and research.
Meningeal lymphatics-microglia axis regulates synaptic physiology [Kim et al., Cell, March 2025]
Dysfunction of the meningeal lymphatic vessels—which transport waste from cerebral-spinal fluid (CSF) to the deep cervical lymph nodes (dCLNs)—has been shown to be well-correlated with various neurodegenerative diseases, particularly those pertaining to memory. Despite the known relationship, the mechanism by which this transport deficiency causes adverse neurological effects is unknown. Kim et al. seek to understand this mechanism, postulating that the disruption of the meningeal lymphatic system will bias the balance between excitatory and inhibitory (E/I) signals in neurons, resulting in deficiencies in global neural computation.
To evaluate their hypothesis, the authors first surgically ligated afferent lymphatic vessels in mice—specifically those that transport to the dCLNs. When these dCLN-ligated mice were presented with a novel object and familiar object in a water-Y-maze experiment, they exhibited reduced a preference for the novel object relative to the control/sham mice, indicating issues in memory formation or retrieval. This observation was also correlated with a decrease in the frequency of miniature inhibitory postsynaptic currents (mIPSC), indicating an excitatory bias in the E/I balance. This result was recapitulated genetically with ablation of the vessels using a VEGF-C/D-trap system.
Due to their established role in responding to changes in the meningeal lymphatic system, the authors then proposed that microglia assisted in mediating the decrease in mIPSC frequency. In dCLN-ligated mice with depleted microglia, they observed the abolition of behavior previously observed in the dCLN-ligated mice. A followup qPCR of the original dCLN-ligated mice revealed a 3.5-fold increase in the expression of IL6. Prior studies have shown that excessive IL6 can decrease inhibitory synaptic responses by altering GABA trafficking, so the authors postulated that the upregulation of IL6 causes the observed frequency decrease in mIPSC. In an experiment comparing IL6 WT and KO dCLN-ligated mice, this claim was validated as the KO mice had greater interest in the novel object than the WT mice.
Finally, to exhibit therapeutic value, the authors demonstrated that enhancing meningeal lymphatic function in aged mice restored the frequency of mIPSC, which originally was 22% lower than that of young mice. Further studies are necessary to fully understand the relevant mechanistic pathway, but the results in this paper lay a solid foundation to build upon – vital for a greater understanding of the underpinnings of biological aging associated cognitive decline and potential means to mitigate or reverse this process.
Notable deals
Merck is betting on a next-gen heart drug, paying $200M upfront to license Jiangsu Hengrui’s Lp(a)-blocking pill, HRS-5346, currently in Phase 2. The deal gives Merck rights outside Greater China, with up to $1.77B more on the table if milestones are met. HRS-5346 joins a crowded field, with Novartis leading the pack—its RNA-based contender is in Phase 3, with key outcome data expected in 2026.
Novo Holdings has co-led Hillstar Bio’s $67 million Series A financing to support the development of next-generation precision immunotherapies for autoimmune diseases. The company put forth in their press release that the funding will go in particular toward supporting their current lead program, TRBV9, paving the way for a clinical proof-of-concept study in axial spondyloarthritis. Other investors in this round include Droia Ventures, Frazier Life Sciences, LifeArc Ventures, and Hummingbird Bioscience.
Character Biosciences has raised $93 million in an oversubscribed Series B round to advance its precision medicine pipeline for degenerative eye diseases, beginning with age-related macular degeneration (AMD). The funding, co-led by aMoon and Luma Group, will support clinical trials for its lead candidates, CTX114 and CTX203, both targeting genetically-defined subtypes of AMD. Character Bio uses a data-driven, AI-powered platform that integrates genomics with clinical data from over 6,500 patients to develop targeted therapies. The company also announced a collaboration with Bausch + Lomb to develop novel AMD treatments.
Epicrispr Biotechnologies closed a $68 million in Series B round ahead of their lead program, EPI-321, entering clinical trials. EPI-321 seeks to treat facioscapulohumeral muscular dystrophy (FSHD) via targeting suppression of aberrant gene DUX4, and is the first epigenetic therapy for neuromuscular indications to enter the clinic. The round was led by Ally Group, with venture nonprofit, SOLVE FSHD, contributing as well.
What we listened to
What we liked on socials channels
Field Trip
Did we miss anything? Would you like to contribute to Decoding Bio by writing a guest post? Drop us a note here.