BioByte 107: bi-paternal mice, long Covid-19 effects on microglia, selective somatic mutation in the liver, and the potential (and potentiation) of rabbits for decoding memory
Welcome to Decoding Bio’s BioByte: each week our writing collective highlight notable news—from the latest scientific papers to the latest funding rounds—and everything in between. All in one place.
What we read
Blogs
It began with a rabbit: Unraveling the mystery of memory [Tim Vernimmen, Knowable Magazine, March 2025]
Imagine a rainy July day in Norway when a simple experiment with live rabbits set the stage for an amazing discovery in biology. Over fifty years later, we still learn from long-term potentiation, or LTP, a process that shows how neurons build stronger connections over time to help us learn and remember.
Back in the early 1970s, neuroscientists Tim Bliss and Terje Lømo were just a couple of curious minds enjoying brunch when they decided to try something new. They sent repeated electrical pulses to rabbit hippocampal neurons and found that these cells began to react more strongly to later signals, sometimes for hours. This breakthrough experiment gave scientists the first real look at how memories might be stored in the brain.
LTP teaches us that the connections in our brain, known as synapses, are not fixed. They grow stronger with repeated use, much like a frequently traveled path that becomes easier to walk. On a molecular level, proteins such as AMPA and NMDA receptors along with the neurotransmitter glutamate work together to boost these signals, making the connection between neurons much more effective.
This discovery in rabbits turned out to have big implications. LTP not only helps explain how we learn but is also linked to issues like memory loss, chronic pain, and anxiety. It reminds us that even simple experiments can lead to answers about some of the most complex parts of our bodies.
Today, the work of Bliss and Lømo still inspires research. Their findings encourage scientists to explore new treatments for neurological problems and deepen our understanding of how the brain works.
Beam base editing therapy gets ‘proof of concept’ in rare lung disease [Ned Pagliarulo, Biopharma Dive, March 2025]
Beam, a pioneer in CRISPR-based base-editing, released positive data from their alpha-1 antitrypsin deficiency (AATD) trial. The therapy (BEAM-302) targets the SERPINA1 gene variant, which, when aberrant, produces a misfolded AAT protein which leads to accumulation; leading to inflammation and cirrhosis of the liver.
For all nine treated patients, results suggest the therapy is effective without any serious side effects. One patient, treated with the highest dose, circulating levels of misfolded protein were 78% lower than baseline after one month.
Alongside the data, Beam announced the pricing of a stock sale. It anticipates a raise of $500M to extend its runway to 2028. Shares dropped due to the offering prices.
Papers
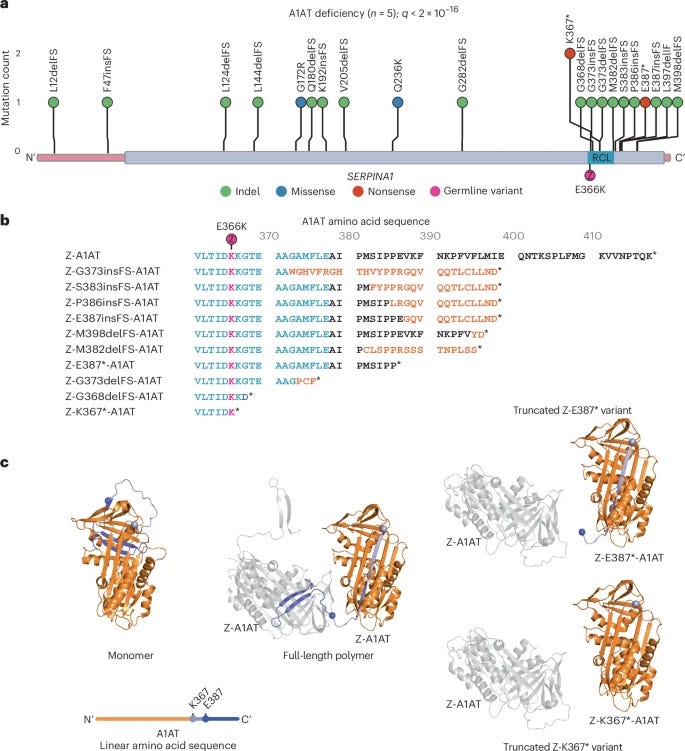
Selection for somatic escape variants in SERPINA1 in liver of patients with alpha-1 antitrypsin deficiency [Brzozowska et al., Nature Genetics, March 2025]
The analysis of somatic mutations is emerging as a powerful tool to understand the selection pressures experienced by cells in both healthy and diseased tissues. By identifying mutations that confer a selective advantage, it is possible to uncover potential therapeutic targets that may help to mitigate disease progression.
This recent study explores the accumulation of somatic mutations in the liver of patients with chronic liver disease that has been caused by alpha-1 antitrypsin deficiency (AATD). The researchers found that somatic mutations in SERPINA1 – the gene encoding alpha-1 antitrypsin (A1AT) – were under strong positive selection in these individuals. Notably, the majority of these resulted in truncated A1AT protein, reducing its ability to polymerize and disrupt the endoplasmic reticulum. The authors suggest that these somatic mutations could give a significant selective advantage to the cells.
Overall, this study and others show that differing pathological processes result in different selection pressures in tissues. Identifying variants that protect against these pathological processes can not only be informative for target identification but also for understanding how the function of these proteins can be manipulated to produce effects that counteract disease mechanisms.
Adult bi-paternal offspring generated through direct modification of imprinted genes in mammals [Wang et al., Cell Stem Cell, March 2025]
A groundbreaking study has achieved a huge milestone in reproductive biotechnology by successfully generating bi-paternal mice—offspring derived from two fathers—that survived to adulthood. This was accomplished by precisely modifying 20 key imprinted loci, addressing a long-standing biological barrier that has made unisexual reproduction impossible in mammals. Imprinted genes, which regulate growth and development in a parent-specific manner, typically prevent embryos from same-sex parents from developing normally. While previous research enabled bi-maternal offspring—mice with two mothers—bi-paternal embryos have historically failed at even earlier stages. By correcting imprinting defects that drive severe developmental abnormalities, including overgrowth and organ malformations, the researchers enabled the birth of viable bi-paternal mice for the first time.
One of the most significant challenges in this process was the development of a functional placenta. Bi-paternal embryos, even with corrected imprinting patterns, could not survive to term due to placental deficiencies. To overcome this, the researchers modified the Sfmbt2 microRNA cluster, a key regulator of placental function, allowing the embryos to develop with a functional placenta. While the survival rate remained low, the successful birth and maturation of some bi-paternal mice represent a major step toward understanding and potentially overcoming the imprinting barriers that limit reproductive possibilities in mammals.
Beyond its implications for reproductive biology, this research holds profound significance for cloning and regenerative medicine. The same imprinting defects that prevent unisexual reproduction also limit the viability of embryonic stem cells and somatic cell nuclear transfer, two foundational techniques in genetic engineering and therapeutic applications. By demonstrating that these defects can be systematically corrected, the study suggests new possibilities for improving cloning efficiency, developing infertility treatments, and advancing stem cell-based therapies—though the ethics and long-term feasibility of unisexual reproduction remains up for debate.
Microglia dysfunction, neurovascular inflammation and focal neuropathologies are linked to IL-1- and IL-6-related systemic inflammation in COVID-19 [Fekete et al., Nature Neuroscience, March 2025]
Prior research has indicated a myriad of neurological phenomena associated with SARS-CoV-2, though the mechanisms underlying these linkages are largely unclear. Evidence has established Covid-19 as a systemic disorder affecting multiple organs and exhibiting strong associations with widespread inflammation. This suggests the implication of the microglia, the main inflammatory immune cell type present within the central nervous system (CNS) parenchyma, as is the case within other cerebrovascular disorders that bear resemblance to Covid-19. Thus, the authors of this study hypothesized that these diverse neurological etiologies arise from microglial dysfunction precipitated by direct viral exposure or associated inflammation at the heterogeneous focal lesions apparent in the brains of patients infected with Covid-19.
This hypothesis was examined via an autopsy platform allowing for analysis of molecular anatomy, proteins, and mRNA transcriptomics of postmortem tissue samples from four brain areas: the gyrus rectus, the temporal cortex, the hypothalamus and the dorsal medulla. The study seemed to find that initial exposure of microglia to viral load triggers a chain reaction to produce interleukin-1 and -6 (IL-1 & IL-6), proinflammatory cytokines that precipitated further immune response and broader inflammation throughout different areas of the brain. Likewise, substantial downregulation of P2Y12R, a critical regulator of microglial immune response, was observed, with particular intensity in microglia occupying closer proximity to cerebral vasculature. Downregulation appeared to be proportional to viral load.
Furthermore, cell-to-cell communication along the observed CX3CR1-CX3CL1 axis, vital for mediating microglia–neurovascular interactions, was significantly impaired due to reduced expression of both proteins. Disruption of essential regulators—P2Y12R and CX3CR1—appeared related to synaptic degradation, and were found to be strongly associated with myelin injury. Overall, affected microglia themselves were found to have undergone acute dysfunction, spatial dislocation, and cell death. Of note, one of the brain areas most severely impacted by these effects was the dorsal medulla, responsible for governing many autonomic functions, though many other areas similarly suffered. Despite heterogeneity of clinical background across patients, relatively homogeneous immune responses were observed on the cellular level, further supporting the central role of microglia in Covid-19-related neuropathologies.
Bonus!: Using Olinks proteomics, authors also trained an AI model to identify Covid-19 presence in patient samples. The model identified eight proteins (EN-RAGE, NMNAT1, CCL20, TGFα, ST1A1, CTSC, CX3CL1 and SCARB2) that it used to identify SARS-CoV-2 viral presence with 97% accuracy.
All of these inflammatory impacts place further stress on an already strained vascular system, with inflammation, microglial dysfunction, synaptic deregulation being shown to persist long after initial infection, even in mild cases and for those who have since recovered. This can lead to significant cognitive impairment, as well as the emergence of new or aggravation of existing psychological disorders, such as depression, anxiety, ‘brain fog’, and autonomic debilitation. Based on the findings in this study, certain targeted anti-inflammatory treatments are suggested to mitigate these effects (i.e. IL-1 antagonists), though this research is still early. Further study is needed to assess the evidently far-reaching systemic effects of SARS-CoV-2 infection and the mechanisms implicated in ‘long COVID’, yet this research makes significant progress toward this end, whilst underlining the criticality of developing therapeutic tools and treatments to combat the lingering and debilitating effects.
Notable deals
Lila Sciences, launched by Flagship Pioneering, is building towards the world’s first scientific ‘superintelligence platform’, combining AI and autonomous labs to accelerate discovery across biological and materials sciences. With $200 million in early funding, Lila aims to scale experimentation, using AI to generate hypotheses, run experiments, synthesise the results and inform the next step. According to the launch release, their early successes include “breakthroughs in genetic medicine, novel therapeutics, green hydrogen catalysts, and carbon capture materials”. Led by top AI and scientific experts, including George Church and Andrew Beam, Lila seeks to redefine the scientific method and drive faster innovation across industries.
Bristol Myers is buying 2seventy bio, Bluebird’s cell therapy spinout, for $286M, but when factoring in cash, the deal values the company at just $102M. This gives Bristol full control of Abecma, its multiple myeloma CAR T therapy, which has struggled to keep pace with Carvykti from Johnson and Johnson and Legend Biotech, a treatment that pulled in nearly $1B last year. Abecma faced early supply constraints, and with another competitor from Gilead and Arcellx on the way, its market position has weakened.
2seventy was spun out of Bluebird in 2021 with the idea that separating cell and gene therapy would allow each to focus, but both struggled. Bluebird burned cash on high costs and slow sales, eventually selling last month to avoid defaulting. 2seventy tried to refocus by offloading its pipeline to Regeneron and Novo but could not make Abecma competitive.
Junction just raised $18M to expand its platform, which connects over 500 wearable devices to labs, making it easier to use real-world health data for diagnostics and care. Originally called Vital, the startup began by standardizing device data and later moved into lab testing. Now, it helps people order and share lab results across all 50 states, handling about a million tests a year.
Founded in London and now based in New York, Junction is betting that the future of healthcare is in the home, not the clinic. With more than 2 million connected devices and 140 healthcare organizations using its platform, including Found and Parsley Health, it is streamlining how data moves between patients, doctors, and labs. The round was led by Creandum, with backing from Y Combinator, Point Nine, and Amino Collective.
What we liked on socials channels
What we listened to
Field Trip
(5:25 is ‘Roma Fade’ — if you’re watching only part of our field trip, it may as well be our favorite of this concert!)
Did we miss anything? Would you like to contribute to Decoding Bio by writing a guest post? Drop us a note here.