BioByte 098: big challenges in protein-design, OpenAI takes clinical trials, how non-neural cells remember and predicting psychiatric disease from social media.
Welcome to Decoding Bio’s BioByte: each week our writing collective highlight notable news—from the latest scientific papers to the latest funding rounds—and everything in between. All in one place.
What we read
Blogs
Google DeepMind releases code behind its most advanced protein prediction program [Catherine Offord, Science News, November 2024]
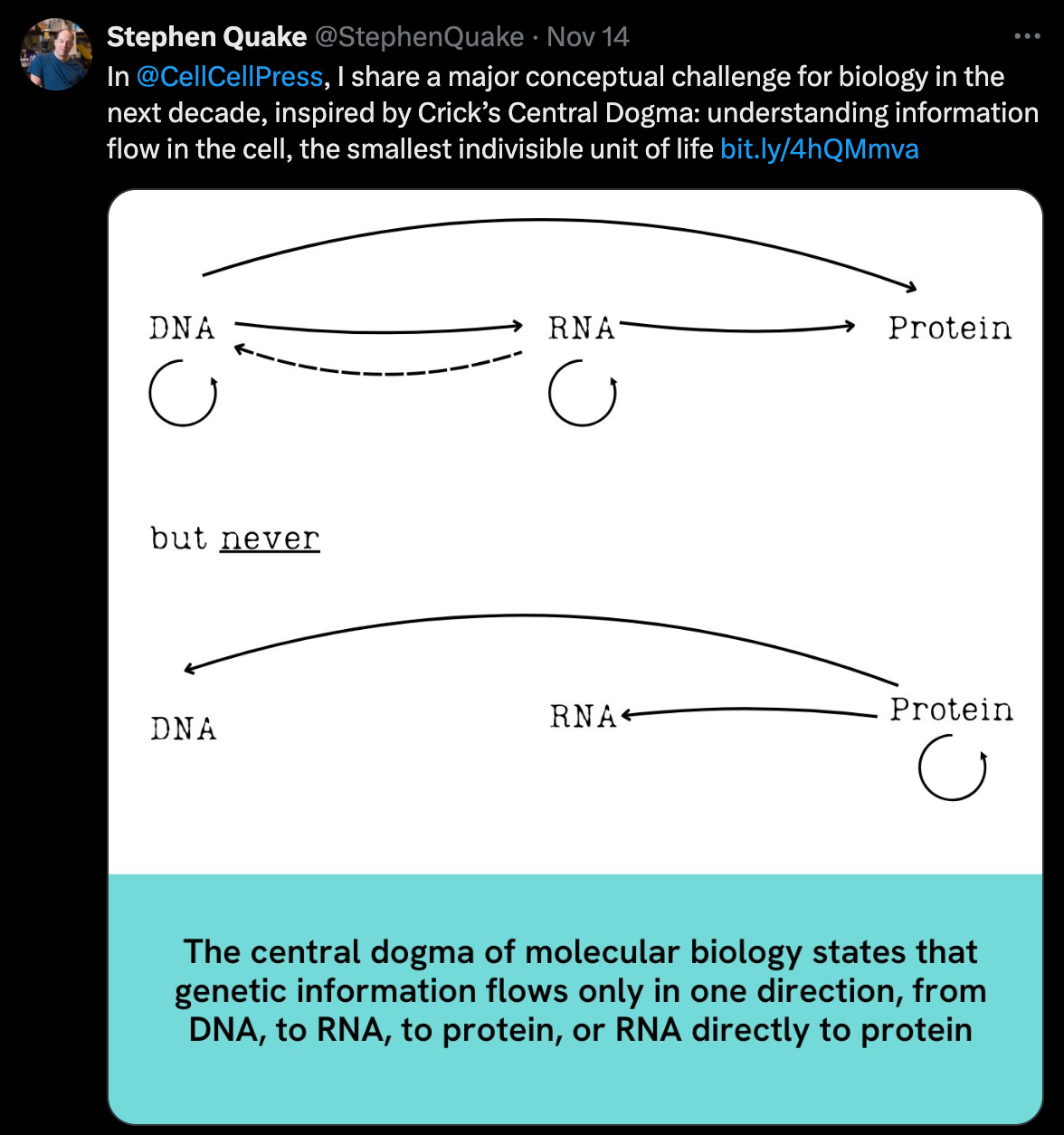
AlphaFold 3 has led the charge for AI-enabled protein structure prediction since its announcement in Nature this past May. This new iteration improves on the prior AlphaFold models, allowing for increased functionality in predicting interactions with DNA, RNA, and other proteins. Despite its eminence for scientific advancement, the model was initially published without its underlying code, contrary to Nature’s editorial guidelines. As of Monday, however, DeepMind publicly released the code on GitHub and is also now offering the weights used to refine the model upon request.
While many within the scientific community have expressed excitement about this long-awaited disclosure, much dismay has followed the initial decision to delay the release of the full code base, citing that this move hindered critical review of the paper far longer than is customary for newly published findings. It also impeded further advancements that come with individual groups building upon and refining the original model, creating a six-month delay for such undertakings which at least one scientist interviewed described as “unacceptable.” Nonetheless, representatives from DeepMind argue that the model was replicable given that several groups including Baidu, Ligo Biosciences, and Chai Discovery did so successfully from the pseudocode initially disclosed in the paper.
Rationale for the delay varied from protecting the interests of DeepMind’s drug discovery spinout—Isomorphic Labs—to concern over biosecurity and ethics, as well as the improvement of their portal interface for easier and more widespread accessibility. One thing is for certain, the ultimate release of the code behind AF3 opens a world of new applications for the prolific tool, though this scenario also raises important questions pertaining to the benefits and barriers of publicly releasing critical methodology of scientific work originating in commercializable industry research.
Five protein-design questions that still challenge AI [Nature, Nov 2024]
With all the hype around AI for drug discovery, what are some key challenges still facing the field?
Building reliable binders - AlphaProteo and RFDiffusion are two tools that can generate a protein binder to a given protein. However, while these binders may attach securely, they don’t always achieve the desired functional outcome. In some cases, though, achieving a specific function may not be essential if the binder serves as a “warhead” component, designed to enhance the effectiveness of another therapeutic.
Designing new catalysts - natural enzymes with similar functions can serve as scaffolds for designing artificial enzymes—like creating one that breaks carbon-silicon bonds. However, starting with natural templates isn’t always the most efficient or optimal approach. It remains to be seen if generative AI models can design functional and efficient catalysts outside of typical scaffolds.
Accounting for conformational changes - proteins aren’t static; they change shape based on environmental factors. Modeling these dynamic conformations remains computationally intensive, with limited data for training models to capture the full range of possible movements.
Creating complex molecular machines - AI has made progress in designing simpler structures, but complex molecular machines, such as motors or cellular transporters, require more sophisticated modeling of multi-component interactions. Human input is still essential to piece together the foundational building blocks AI creates.
Learning from mistakes - Models rarely get feedback on from failures or negative data, which hinders iterative improvement. Closing the loop between computational design and experimental testing is key to advancing reliable protein design.
Sanofi, OpenAI, Formation Bio debut patient recruiting tool, will use in Phase 3 studies [Andrew Dunn, Endpoints News, Nov 2024]
Sanofi, OpenAI, and Formation Bio have unveiled Muse, their first AI-powered tool designed to streamline patient recruitment for clinical trials, addressing a costly bottleneck in drug development. Muse, built on a customized version of GPT-4o, can segment patient groups, suggest tailored engagement plans—such as virtual support groups—and generate content like social media posts to promote trials, all while incorporating compliance checks. This collaboration, announced in May, aims to tackle broader challenges in drug development, including protocol writing and strategic planning. Sanofi is integrating Muse into its upcoming trials, including Phase 3 multiple sclerosis studies, as part of its AI-driven transformation under CEO Paul Hudson. Formation Bio also plans to leverage Muse as it transitions from contract research to drug development, supported by a recent $372 million funding round. While Muse enhances efficiency and reduces costs, its outputs are reviewed by human experts, ensuring accuracy and continuous improvement.
Papers
The massed-spaced learning effect in non-neural human cells [Kukushkin et al., Nature Communications, Nov 2024]
A recent paper from Thomas Carew’s lab at NYU examines a well-known phenomenon in learning and memory called the "massed-spaced effect" - the observation that learning or training spread out over time (spaced) is more effective than the same amount of training delivered all at once (massed). Think of how studying for an exam over several days is typically more effective than cramming the night before.
While this effect has been primarily studied in neural circuits, it was hypothesized if it might be more fundamental, existing at the level of basic cell signaling that all cells share. To test this, the authors created two types of human non-neuronal cell lines. These cells were engineered to produce a glowing protein (luciferase) when certain cellular signaling pathways were activated. When given four brief pulses of stimulation with 10-minute gaps, the cells showed stronger and more persistent responses compared to one continuous stimulation. This enhanced response required two key proteins: ERK (a cellular signal messenger) and CREB (which controls gene activation). Blocking either protein eliminated this enhanced response to spaced stimulation.
The significance is that this demonstrates fundamental features of memory formation don't require specialized nerve cells but can arise from basic cellular machinery present in many cell types, such as kidney cells. This opens new ways to study memory formation at the molecular level and might lead to new approaches for treating memory-related disorders.
Beyond the blood: expanding CAR T cell therapy to solid tumors [Uslu & June, Nature Biotechnology, 2024]
In a relatively short period of time, the field has continued to iterate and improve the efficacy of CAR-T cell therapies. While they traditionally achieved significant success in blood cancers, recent data has demonstrated breakthroughs in solid tumors. The most notable innovations include the development of third-generation CAR designs incorporating multiple co-stimulatory domains (CD28 and 4-1BB), which have shown improved efficacy particularly in solid tumors such as neuroblastoma with a 63% response rate. Additionally, the implementation of novel targeting strategies, such as dual-targeting CAR T cells and local administration methods, has led to rapid tumor regression in glioblastoma patients within 24-48 hours of treatment.
Several technical innovations across the continuum of treatment have improved outcomes. The introduction of lymphodepleting chemotherapy regimens combining fludarabine and cyclophosphamide has significantly improved CAR T cell persistence. Multiple CAR T cell infusions, rather than single doses, have shown better efficacy, with some trials allowing up to four sequential treatments. Patient selection strategies have also evolved, with some trials now requiring specific threshold levels of target antigen expression (≥40% of tumor cells) to optimize response rates.
Synthetic biology has made its way here, with new engineering strategies including bispecific and trispecific CARs operating as "OR gates" to combat tumor heterogeneity, CAR T cells engineered to secrete additional therapeutic molecules, and the incorporation of safety mechanisms like inducible suicide genes. Alternative cell types, such as natural killer cells and γδ T cells, are being explored as CAR carriers, offering additional targeting mechanisms through their natural receptors, offering a diversity of options.
Recent innovations in trafficking and persistence include the development of chemokine receptor-modified CAR T cells to enhance tumor infiltration, and the use of CAR T cell-amplifying RNA vaccines. Novel approaches to overcome the hostile tumor microenvironment include CAR T cells engineered to express IL-7 and CCL19/CCL21 to recruit additional immune cells, and cells designed to modify the tumor stroma through the secretion of enzymes like heparanase or hyaluronidase. The field is also exploring combination strategies with radiation therapy to remodel the tumor microenvironment and enhance CAR T cell efficacy.
Identifying signals associated with psychiatric illness utilizing language and images posted to Facebook [Birnbaum et al., Nature Schizophrenia, 2020]
Why it matters: psychiatric illness and its diagnosis is challenging given its complex etiology and symptomatic variability. The authors of this paper were able to differentiate schizophrenia spectrum disorders (SSD) and mood disorders (MD) using Facebook data alone over a year in advance of hospitalization. The authors opine that integrating this data with clinical information could improve clinical decision-making.
In this older paper, the authors collected over 3 million Facebook messages and 142 thousand images across 223 participants with schizophrenia spectrum disorders (SSD), mood disorders (MD) and healthy volunteers (HV). The team built classifiers that distinguished SSD and MD from HV, and SSD from MD.
The following features were significantly correlated with conditions:
“SSD used more (P < 0.01) perception words (hear, see, feel) than MD or HV
SSD and MD used more (P < 0.01) swear words compared to HV.
SSD were more likely to express negative emotions compared to HV (P < 0.01).
MD used more words related to biological processes (blood/ pain) compared to HV (P < 0.01).
The height and width of photos posted by SSD and MD were smaller (P < 0.01) than HV.
MD photos contained more blues and less yellows (P < 0.01).
Closer to hospitalization, use of punctuation increased (SSD vs HV), use of negative emotion words increased (MD vs. HV), and use of swear words increased (P < 0.01) for SSD and MD compared to HV.”
Notable deals
Novartis has partnered with Schrödinger, providing $150 million upfront to collaborate on a set of targets outside of oncology. Under the agreement, the two companies will jointly advance these targets through discovery, after which Novartis will assume responsibility for further development. Schrödinger could receive up to $892 million in milestone payments tied to R&D and regulatory achievements, and up to $1.38 billion for potential commercial milestones. Additionally, a separate agreement includes an undisclosed increase in Novartis’s investment in Schrödinger’s software platform.
Vesalius and GSK have entered a strategic alliance to jointly discover and develop innovative treatments for Parkinson’s disease. This collaboration leverages Vesalius’s machine-learning platform, which integrates insights from phenotypic cellular data and multi-gene circuits to identify novel biomarkers and therapeutic interventions. The partnership will focus on advancing Vesalius’s preclinical small molecule program, with the option to progress additional programs that emerge through their joint efforts. GSK will provide $80 million in upfront and equity payments, along with milestone payments contingent on continued progress and success.
Metsera raises $215M, just half a year after its first $290M round. The fresh Series B funds will be used to progress its long-acting GLP-1 injectable, oral GLP-1 and amylin analog (to reduce muscle loss) through Phase II trials. The competition in the obesity drug development space is fierce, speed and capital are key as biotechs rush to market with their improved offerings.
What we liked on socials channels
Field Trip
Reminiscing summer days…