BioByte 085: monte carlo models in drug discovery, we don't know how antidepressants work, single cell atlas for Alzheimer's Disease, extending lifespan with IL-11, impact of diet on the microbiome
Welcome to Decoding Bio’s BioByte: each week our writing collective highlight notable news—from the latest scientific papers to the latest funding rounds—and everything in between. All in one place.
We hope you all enjoyed last week's AI x Bio Summit. We certainly found seeing everyone together extremely energizing. And now, for some juicy content.
What we read
Blogs
We don’t know how antidepressants work [Chemistry World, June 2024]
Depression is a complex condition with both social and biochemical roots. While antidepressant drugs like SSRIs (selective serotonin reuptake inhibitors) have been widely used, their exact mechanism of action, which patients benefit / which don’t and long-term effects remain uncertain.
Recent research by Hashemi and colleagues offers a new way to study depression using organoids – miniature, simplified organs grown from a person's own cells. By integrating these organoids into chips, researchers can monitor electrical currents across neurons and infer levels of neurotransmitters like serotonin and histamine. This approach could help us understand how antidepressants work and tailor treatments to individual patients.
However, the link between serotonin and depression is not as clear-cut as once thought. Early studies focused on the serotonin receptor, which SSRIs target, but some research has questioned the direct relationship between serotonin levels and mood. Additionally, the long-term effects of SSRIs on brain chemistry are still under investigation.
One promising avenue of research involves a protein called BDNF (brain-derived neurotrophic factor), which plays a crucial role in neuroplasticity – the brain's ability to adapt and change. Studies by Heliskinki’s Enero Castrén's team suggest that antidepressants may work by binding to the TrkB receptor, which recognizes BDNF. This finding could lead to the development of new antidepressants that target BDNF signaling pathways more directly.
1H 2024 Venture Healthcare Report [Jonathan Norris et al., HSBC Innovation Banking, July 2024]
An HSBC Innovation Banking analysis of the venture deals in the first half of 2024 indicates a significant rebound from a 2023 that was dominated by insider-rounds. The investment increase in 1H 2024 is partially driven by a greater number of high-value, first-financing investments that were particularly concentrated in biopharma and med devices.
Across all venture deals in biopharma, oncology and platform indications led the way with a combined $6.8B across 138 deals. Additionally, metabolic and auto-immune indications have already surpassed the total investments in these areas in 2023 by 130% and 40% respectively. First-financing investments followed a similar trend, with all indications except Orphan-Rare either surpassing or close to surpassing the 2023 numbers, albeit often with a slightly reduced deal count.
Although med device investments showed less total growth than biopharma, the first-financing deals in this sector were equally impressive. Neurology first-financing has almost tripled from 2023, and NIM first-financing investment dollars have also already increased by 125%. Of the other med device indications, vascular and respiratory were the only two that showed lower first-financing investment levels. The report also details a rise in earlier stage deals in healthtech, along with the slight decrease in diagnostics investments from Q1 to Q2, though 1H 2024 was still an overall increase from 2H 2023.
Lessons from Monte Carlo Models: Why Drug Development is Hard [Drug Baron, February 2024]
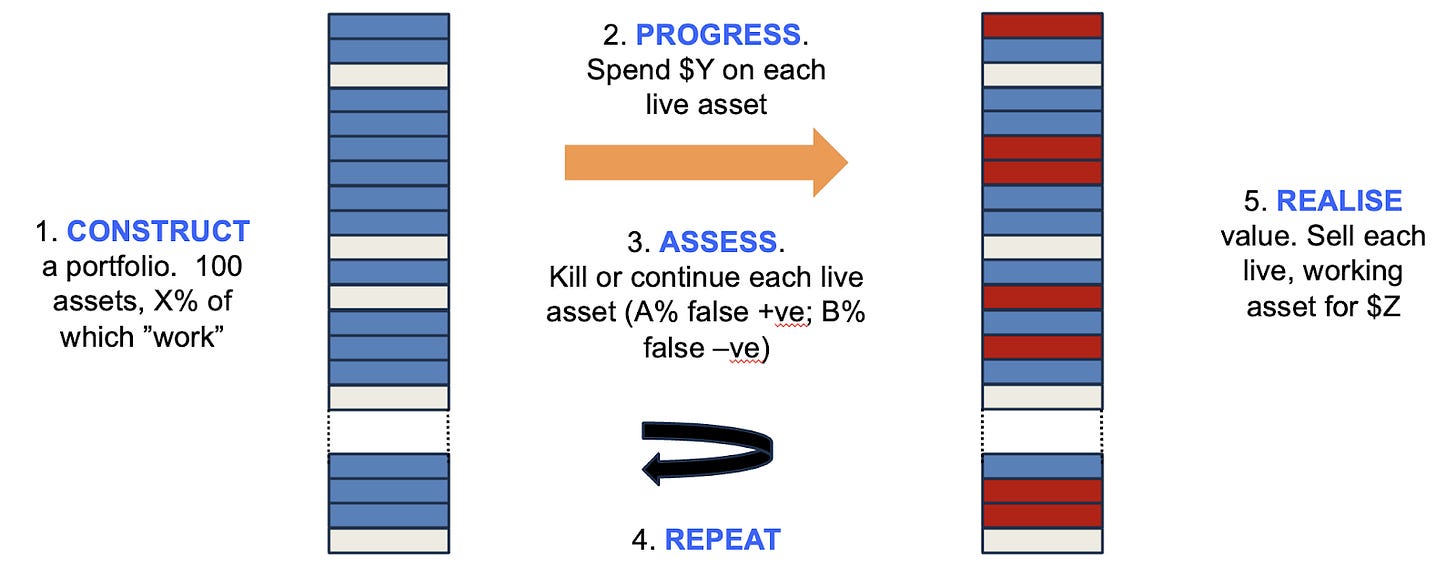
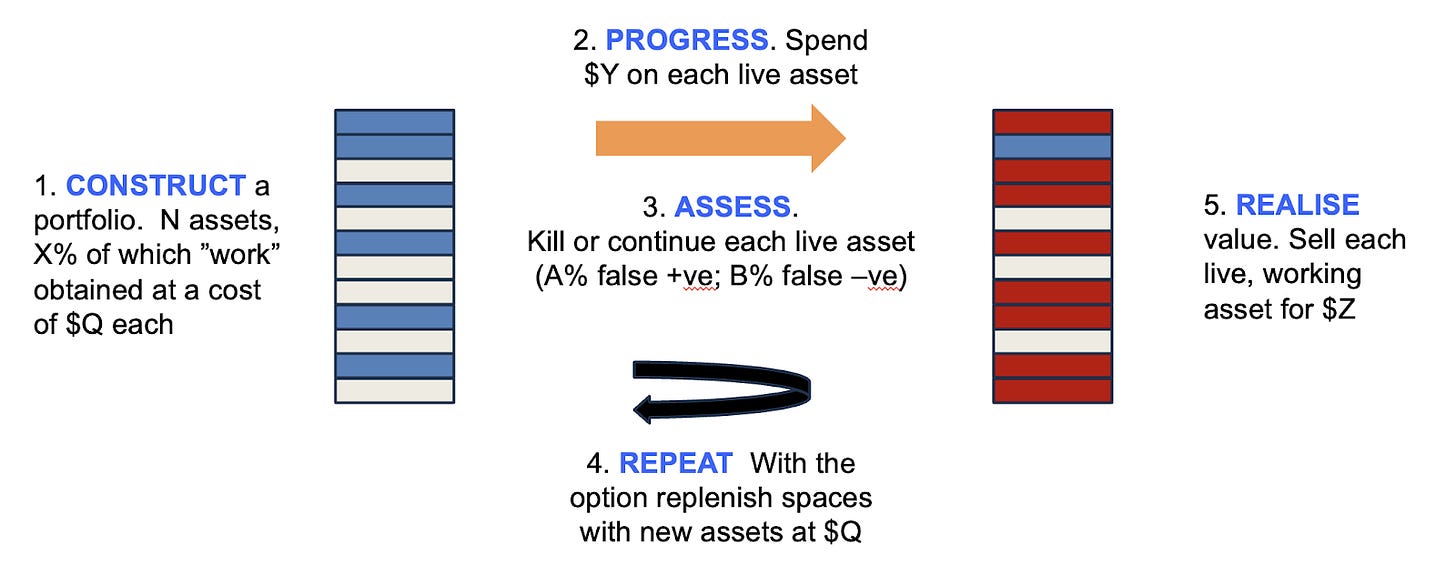
The author here describes the lessons from using Monte Carlo simulations to its asset-centric investment model at venture firm Medicxi.
The firm used Monte Carlo simulations to model returns based on an early-stage synthetic portfolio with different conditions (% of assets that work, $ spent per asset, false positives and false negatives and $ return per asset that works). He mentions “What this taught us that returns are most sensitive to costs, particularly during the early iterations (such that it rarely makes sense to pay extra for more information, even if it modestly improves the quality of the decision filter). It also showed us that stringency (killing more things, even at the cost of more false negatives) is the critical parameter.”
In a late-stage portfolio, however, the cost of progressing each asset is irrelevant, and the most important parameter is the fraction of good assets in the initial mix. Returns are also improved by lowering the stringency of the filter (by allowing assets to be developed after appearing to fail in case the decision was a false negative, and paying for even a slight improvement in the quality of the decision filter is a great investment.
Academic Papers
Single-cell multiregion dissection of Alzheimer’s disease [Mathys et al., Nature, July 2024]
Alzheimer's disease (AD) is the leading cause of dementia worldwide, but the cellular pathways underlying its progression across brain regions are not well understood.
In a new paper from the labs of Manolis Kellis and Li-Huei Tsai, the groups aimed to create a comprehensive single-cell atlas of multiple brain regions affected at different stages of AD to gain new insights how specific neuronal cells and circuits are susceptible (or resilient) to disease.
The team performed single-nucleus RNA sequencing on over 1.3 million cells from 6 different brain regions in 48 individuals with and without AD. They used advanced computational methods to identify cell types, gene expression programs, and differences associated with AD pathology. The authors identified 76 distinct cell types across the brain regions, including some region-specific neuronal subtypes such as thalamus-specific inhibitory neurons and hippocampus-specific excitatory neurons, highlighting the diversity of cell types and brain regions affected in AD.
The researchers investigated factors associated with cognitive resilience in Alzheimer's disease by analyzing gene expression in different cell types across brain regions. They found that astrocytes expressing genes involved in antioxidant activity, choline metabolism, and polyamine biosynthesis were strongly linked to preserved cognition, even in the presence of significant pathology. This finding aligns with previous research on the benefits of choline supplementation and suggests a potential role for spermidine, an antioxidant supplement. The team validated these predictions by observing increased expression of these genes in brain tissue samples from cognitively resilient individuals.
The study revealed that neurons expressing Reelin, a protein crucial for brain development and function, are particularly vulnerable in Alzheimer's disease. This vulnerability was observed in both excitatory and inhibitory neurons, especially in the entorhinal cortex, an area affected early in AD. The findings were validated in human brain tissue and mouse models, showing a significant decrease in Reelin-positive neurons in AD conditions. This discovery is important as it identifies specific cellular targets for potential therapies, provides insights into the mechanisms of neuronal loss in AD, and suggests that changes in Reelin-expressing neurons could serve as an early marker of disease progression.
Perhaps most excitingly, the data were released publicly into a database and interactive cell browser, so we expect to see many more interesting discoveries leveraging these data in the future.
The interplay between diet and the gut microbiome: implications for health and disease [Ross et al., Nature Reviews Microbiology, July 2024]
Why it matters: it is long known that diet contributes to the composition and function of the gut microbiome. However, the links between diet and nutrition with the gut is not well characterized and is thus difficult to act on. This review examines several dietary protocols and characterizes its influence on the gut microbiome, paving the conversation for precision nutrition and microbiome-based therapies.
Diet has a strong influence on shaping the gut microbiome. This review investigates various diets including the mediterranean diet, high-fiber diet, ketogenic diet, Western diet, high-protein diet, and plant-based diet. Key findings show the mediterranean diet being linked to beneficial changes like lower inflammation and increased short-chain fatty acid production, high-protein diets are shown to increase protein-fermenting bacteria which may lead to harmful metabolites, and a high-fiber diet is shown to promote healthy bacteria. Such features can be linked to metabolic and cardiovascular disorders. For example, high protein/low fiber diets may translate to higher risk of inflammatory bowel disease. The reviewers also found non-western populations to have richer microbiome, which may be explained by the processed nature of the average western diet. While we understand the impact of certain diets on the microbiome, it is unclear how to put the information into practice from a clinical and therapeutic standpoint. Future larger-scale omics studies should be undertaken to understand how precision nutrition can be tailored to the individual scale for more effective therapies.
Inhibition of IL-11 signaling extends mammalian healthspan and lifespan [Widjaja et al, Nature, 2024]
Several pathways have been identified that regulate lifespan across various species, including those of mTORC1, IGF1-insulin, ERK, STK11, and more. Often, these pathways are modulated over the course of aging, leading to phenomena including inflammation, senescence, and mitochondrial dysfunction. In particular, inflammation has been identified as a critical node in the aging process, as decades of research have shown that inhibition of canonical pathways such as NF-kB and JAK-STAT are directly implicated. Here, the authors posit that IL-11 (a pro-inflammatory and fibrotic cytokine) promotes age-related pathologies and a reduction in lifespan, and is rooted in the observation that IL-11 activates ERK and JAK pathways, is upregulated in older individuals, and is increasingly implicated in senescence.
In aging mice, the authors appreciated increased levels of IL-11 and related signaling pathway in various tissues; they then modulated IL-11 activity via genetic knockout of the molecule itself or receptor subunits, or via a neutralizing antibody. They found that mice with inhibited IL-11 activity demonstrated signs of improved health at older ages (such as ↑ lean tissue, reduction of other pro-inflammatory cytokines, reduced fat synthesis, improved glucose tolerance, and decelerated age-related muscle loss). Impressively, pharmacological inhibition or deletion of IL11 led to an increased lifespan in mice. Of note, given that most mice in the lab die of cancer, these results suggest that IL-11 (and broadly, inflammation) is implicated in cancer-related processes. This work is exciting as it adds to the growing body of work that inflammation plays a critical role in the aging process and the development of age-related diseases. The findings suggest that targeting specific inflammatory pathways, such as IL-11 signaling, could potentially slow down the aging process and improve healthspan in later life. As research continues to unravel the mechanisms underlying inflammaging, it is becoming increasingly clear that managing chronic inflammation will be a key factor in extending both lifespan and healthspan in the future.
What we listened to
Notable Deals
Flagship’s latest creation, Abiologics, launches with $50M: the new biotech will use generative AI and synthetic building blocks to design synthetic biologics (Synteins) which overcome typical issues encountered by natural biologics (e.g. stability).
Ex-J&J execs raise $165M for Third Arc Bio to move three multi-specific antibodies for solid tumours and I&I into the clinic.
Shokat’s latest biotech, Montara, launches with an $8M seed: the biotech plans to use a two-drug combination to tackle ‘undruggable’ neurological targets whilst sparing toxic effects in the periphery.
GSK and Flagship: the pair will commit up to $150M in funding to use GSK’s disease area (immunology & respiratory) and development expertise to advance initial work from Flagship’s 40+ portfolio companies. For each project advanced, GSK is will pay a series of milestones. The partnership structure is similar to that of Flagship and Pfizer.
Brenig Therapeutics closes $65M Series A backed by Orbimed and NEA to bring its LRRK2-targeting neurology compound to clinical trials.
Boehringer will pay up to $1.3B for pre-clinical immune checkpoint inhibitor biotech Nerio
What we liked on socials channels
Events
Some pictures from last week’s Summit at the NYSE!
Field Trip
Did we miss anything? Would you like to contribute to Decoding Bio by writing a guest post? Drop us a note here or chat with us on Twitter: @ameekapadia @ketanyerneni @morgancheatham @pablolubroth @patricksmalone