BioByte 084: A history of Regeneron, 3D genome preserved in wooly mammoth skin, assessing LLMs for biology, technology to overcome the cold chain
Welcome to Decoding Bio’s BioByte: each week our writing collective highlight notable news—from the latest scientific papers to the latest funding rounds—and everything in between. All in one place.
Are you stranded in some airport due to the CrowdStrike outage? Did you finish hacking into Amazon’s central database 12 hours ahead of schedule and don’t know what to do with your life? Well behold as we got some SCIENCE to keep you preoccupied.
What we read
History of Regeneron Pharmaceuticals: 35 Years of Innovation [Regeneron]
This week we went through Regeneron’s exceptional history. Some key highlights:
1988: How Regeneron’s founder Leonard S Schleifer raised its first $1M: “I got to meet George and he struck me as a guy who actually did want to build something, who respected science, who really would let us run, who respected the fact that we had really smart people we were trying to bring around. And wasn't trying to be like a super ego venture capitalist like you find today, who think they know more about the science than anybody else, and they're going to decide what you work on. They're going to tell you how to do it. He wasn't that type of a guy. He was a very, very creative, helpful on the business side, super helpful and helping me understand some of these things. And he and I finally went to a Chinese restaurant and we literally scratched out a deal on the terms that he'd give me a million dollars on the back of a napkin”
1991: REGN IPOs on the NASDAQ, raising $91.6M
1992: Clinical development of first drug, a neurotrophic factor, begins
1997: The neurotrophic factor drug does not achieve its primary endpoint on its Phase 3 trial
2000: Rilonacept begins clinical exploration
In the early 2000s, Regeneron generated one of the first “knockout” mice in biotech history, which allowed them to study how certain genes (or the absence of certain genes) affect health and disease. Their Veloci suite of platforms enables high throughput screening and validation of antibodies
2004: Aflibercept begins clinical development
2006: Sarilumab, REGN’s first fully human antibody, enters clinical development
2008: Rilonacept is approved by the FDA and commerciailzed as Arcalyst
2011: Aflibercept is approved by the FDA and commerciailzed as Eylea
2015: FDA approves alirocumab (Praulent)
2017: FDA approves dupilumab (Dupixent) and sarilumab (Kevzara)
2018: FDA approves Libtayo (cemiplimab-rwlc)
2020: The FDA approves Inmazeb (atoltivimab, maftivimab, and odesivimab-ebgn) for ebola virus
2021: Regeneron and Intellia announce first-ever clinical data supporting the safety and efficacy of in vivo CRISPR genome editing
2022: REGN acquires IO-focused Checkmate Pharmaceuticals
2023: The FDA approves Veopoz (pozelimab-bbfg)
Closing the gender health gap is a $1 trillion opportunity [Kelle Moley, Nature Biopharma DealMakers, May 2024]
Research on women’s health has historically been disproportionately lacking and critically underfunded. Despite women comprising nearly 50% of the global population, a mere 1% of R&D funding targets non-cancer-related women’s health.
While greater equity and reform in this area have long been called for by many, a report by the World Economic Forum and the McKinsey Health Institute released in January 2024 strengthens this case by finally quantifying the economic and human costs of this disparity for the first time. Since the report’s release, international action has taken place with the formation of the Global Alliance for Women’s Health and the announcement of a $12 billion investment in women’s health research from the Biden Administration in the US. Additionally, PE & VC funding of $2.2 billion in the last 4 years is showing increased interest in the space.
Though increased investment is positive and necessary, it constitutes only part of the solution. The author calls for greater initiative and accountability from pharmaceutical companies in furthering women’s health innovation, highlighting specific changes that need to occur such as: increasing representation and visibility of women researchers particularly in leadership positions as well as within clinical trials, facilitating greater education and collaboration between key stakeholders, and leading by example to create equal and inclusive platforms in line with ESG commitments.
Should the status quo of pharma companies neglecting investments into women’s health be maintained, they may find themselves left behind as others take advantage of such a ‘high-potential market.’
Three-dimensional genome architecture persists in a 52,000-year-old woolly mammoth skin sample [Sandoval-Velasco et al., Cell, July 2024]
Elucidating the complex and dynamic 3D configuration of the genome is critical to fully understand gene expression patterns. Techniques such as Hi-C are typically used to understand this nuclear architecture.
However, such techniques have been difficult to apply to ancient samples due to the surviving DNA fragments being too short and diffuse to map accurately with standard Hi-C. Without an understanding of the 3D genome, it may be difficult to fully understand what genes are typically expressed (and where) in ancient species such as the mammoth, making it more difficult to understand how ancient physiological traits are determined.
Baylor College of Medicine’s Cynthia Estrada theorized that since water and bacteria are typically the culprit for the degradation of ancient DNA samples, a dehydrated sample of ancient genome may preserve more of the genomic information regarding architecture. On successfully finding such samples from Siberian Mammoths, scientists from the Aiden Lab developed a technique called PaleoHi-C (Figure 1) to determine what genome architecture was preserved in this sample and what this means for the physiological traits of the mammoth.
The researchers were able to identify several hallmark genomic architectural elements such as chromosome compartments, loops and domains. Using this they were able to accurately determine the activity of various mammoth skin genes and showed that 96% of the genes have activity states that match those in elephant, but 4% that don’t. Key differences were found in genes for hair follicle development. This makes sense given the woolliness of mammoth skin.
Measuring Capabilities of Language Models for Biology Research [Laurent et al., ArXiv, July 2024]
FutureHouse, a nonprofit AI-for-science research lab, published a set of benchmarks and evaluations to aid the development of LLMs and AI agents for automating and augmenting scientific research. Benchmarks across 8 categories (see figure below) were released, including evaluations involving the extraction of information from the scientific literature, reasoning about scientific figures and tables, and designing biological protocols.
Widely used LLMs such as Claude 3.5, GPT-4, and Llama 3 were tested on the benchmarks, but did not perform well. All models fell significantly short of human performance across all tasks, except Claude 3.5 Sonnet on TableQA (reasoning about tables from scientific papers). A couple key observations: models performed poorly on visual reasoning tasks such as interpreting scientific figures (perhaps surprising given recent advances in multi-modal LLM capabilities), and LLMs struggled in tasks like extracting information from the scientific literature because they didn’t have the requisite tools (such as the ability to search the web/PubMed, code, and other plug-ins). A key area of focus going forward will be building the necessary software infrastructure required for LLMs to leverage external tools for scientific tasks.
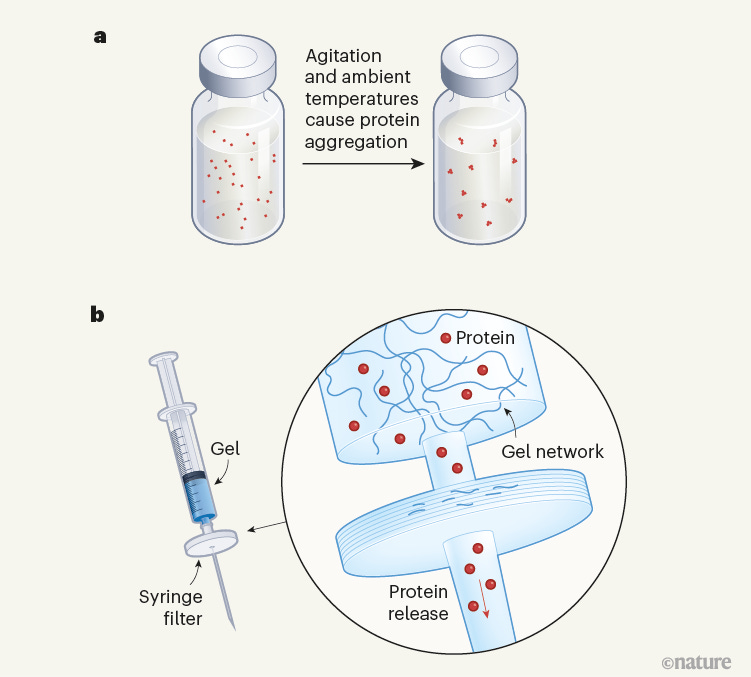
Mechanical release of homogenous proteins from supramolecular gels [Bianco et al., Nature, 2024]
One of the biggest challenges at the intersection of healthcare delivery x therapeutics x global health is navigating the cold chain – where therapeutic medicines (such as proteins) are often temperature sensitive and require immense infrastructure, logistics, and technologies to deliver medicines to patients. This is a heroic task, and necessitates a coordinated effort across constituents to ensure people get the therapeutics they need; however, the cold chain often breaks down, restricting access to life-saving medicines in some regions of the world.
The authors report a gel system for storing proteins that avoids use of additives and stabilizers and enables freedom from the cold chain. They use a small peptide as a gelator, which self assembles instantaneously to form a hydrogel-like substance at 19C and near-neutral pH. They found that when proteins are added to said solution, these become trapped in the gel fibre network, and were readily retrievable using a filter and syringe. The gel itself is crushed by the syringe pressure, is removed by the filter, and only the proteins pass through. They found gelation conditions that recapitulate insulin concentrations comparable to those of commercial formulations – of note, they demonstrated that insulin from the gel that was subject to adverse conditions (agitation and quite elevated temperature for 6 hours) was recoverable with minimal aggregation. They also stored another large protein, β-galactosidase – even after the protein was incubated for 7 days at 50C, the protein retained 97% of its biological activity when recovered as a solution. Additionally, 100% active β-gal was recovered from a gel that was sent through the UK postal service - strongly suggesting that their technology can help address challenges with the cold chain.
The authors’ work holds significant potential for storing therapeutic proteins and rapidly retrieving them with a simple syringe + filter. Although only demonstrated with proteins, this technology holds promise for stabilizing vaccines that also require cold chain management. This is a new scalable method for storing and safely transporting biologics, which could enable effective delivery of therapeutics in under-resourced settings.
What we listened to
Notable Deals
Pfizer, Nvidia and ThermoFisher back CytoReason with $80M to build up more molecular and clinical data to expand its computational models to new diseases
Scorpion Therapeutics raises a $150M Series C to advance its precision oncology pipeline through clinical trials. The company’s lead asset is a wild-type-sparing PI3Ka inhibitor for breast cancer and is currently at phase ½.
Novo Holdings, J&J-backed Asceneuron raises $100M to take Alzheimer's asset into phase 2
SciRhom raises $70M for a new immune disease drug indicated against rheumatoid arthritis and inflammatory bowel disease, having recently showed preclinical experimental success
What we liked on socials channels
Events
See you at the AI x Bio Summit next Thursday! Thank you to all who registered - we’re excited for all of the great discussions to be had.
Field Trip
Did we miss anything? Would you like to contribute to Decoding Bio by writing a guest post? Drop us a note here or chat with us on Twitter: @ameekapadia @ketanyerneni @morgancheatham @pablolubroth @patricksmalone