BioByte 060: astrocyte plasticity, a foundation model for RNA structure, improved confidence in biotech activity in '24, predicting therapeutic perturbations, isomorphic's deals
Welcome to Decoding Bio’s Weekly BioByte, where we discuss the latest scientific advancements, deals, and people building at the intersection of tech x bio. Happy decoding!
We’re back! Can we still say Happy New Year?
We have so much in store for 2024, all of which will be unveiled over the next six months: from events and reports to something completely new. Make sure to subscribe to not miss any of the updates. But for now, let’s dig into some scrumptious content.
What we read
Blogs
Let the Good Times Roll Again: VCs Confident in Biotech Activity for ‘24 [Tyler Patchen, Biospace, Jan 2024]
Patchen reports on biotech sentiment at JPM in this article, highlighting the positive attitude of biotech investors, a stark change from last years’ conference. Bankers are predicting a rally from the last two years of tough news for the biotech industry. Much of this sentiment comes from the last two months—biotech saw a flurry of M&A activity in November and December of 2023, which Patchen reports as the first time that much activity had been seen since 2002. The IPO market is also opening up, though raise size and valuations are compressed from what we’re used to. Investors also warn that the funding process is taking much longer, even up to a year, which is consistent with what the market has shown the last few months.
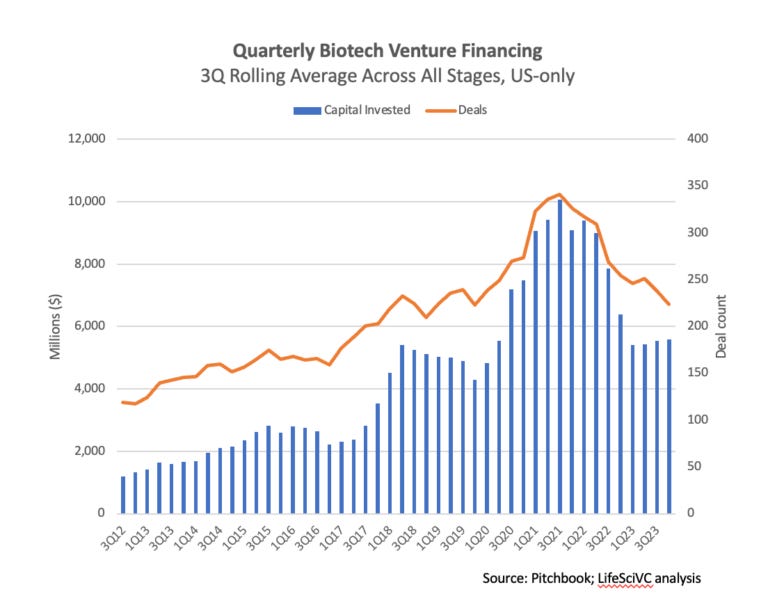
Biotech Venture Ecosystem: Quick Health Check [Bruce Booth, Life Sci VC, January 2024]
In his latest blog post, Bruce Booth provides an update on the biotech venture ecosystem. Overall, the biotech sector is showing positive momentum, with venture funding stabilizing at around $5 billion per quarter. Key trends include a slowdown in new startup creation, continued prevalence of mega-rounds over $100M, and robust private round valuations despite market turbulence. The venture ecosystem is adjusting after a period of rapid startup formation, focusing more on later-stage funding. Despite slower diligence and closing timelines, solid scientific ideas with strong teams are still attracting venture financing. The sector is healthier and more disciplined compared to the previous euphoric or challenging phases, poised for continued growth in 2024.
Techbio is a speciation event [Jacob Kimmel, Creode, Dec 2023]
Jacob Kimmel, Co-Founder of New Limit, describes how he’s recently become convinced that there are unique properties at techbio firms “that constitutes a speciation event from the parental biotech strain.” The whole blog is worth a detailed read, but his points can be summarized as:
Biotechs choose problems with median outcomes using prior knowledge
Techbios choose to tackle hypothesis spaces that maximize expected value and employ in silico models to make them tractable
Academic papers
Injury-specific factors in the cerebrospinal fluid regulate astrocyte plasticity in the human brain [Sirko et al., Nature Medicine, December 2023]
Why it matters: New data demonstrate the role of astrocytes in forming clusters of neural stem cells called neurospheres in response to blood-brain barrier injury, suggesting a mechanism for the regeneration of new brain tissue.
We covered the role of astrocytes (the most abundant non-neuronal glial cell in the brain) in neuroinflammation back in BioByte 057. A recent Nature Medicine paper details another important role of astrocytes: neurogenesis in response to brain injury. One of the primary roles of astrocytes in the healthy brain is to maintain the integrity of the blood-brain barrier (BBB), a semipermeable barrier of endothelial cells that regulates the transfer of substances into the brain. The authors found that after a range of BBB injuries (stroke, TBI, and cerebral caveroma) in human brain tissue from patients undergoing neurosurgery, astrocytes near the site of injury produced a protein called Galactin-3. Galactin-3 helped induce the formation of neurospheres, a cluster of neural stem cells that can generate neurons and astrocytes and potentially regenerate new brain tissue (check out the video below to see neurospheres growing in the hippocampus. h/t BrainPost).
Interestingly, the cerebrospinal fluid (CSF), a fluid that surrounds the brain and spinal cord, seems to regulate astrocyte behavior and neurosphere formation by transporting injury-specific factors. This is therapeutically relevant as it is relatively straightforward to access the CSF (intrathecal administration is commonly done for chemotherapy and anesthesia, for example) relative to deep brain structures, and CSF manipulation or delivery of therapeutics could be targeted for brain repair and regeneration.
ATOM-1: A Foundation Model for RNA Structure and Function Built on Chemical Mapping Data [Boyd et al., bioRxiv, Dec 2023]
Why it matters: many of the therapeutic properties of RNA therapeutics (ASOs), siRNAs, and RNA editing therapies) and small molecules targeting RNA are mediated by structure. Experimental structure elucidation is difficult, especially for RNA, so computational models are critical to optimize and design new RNA-based therapies.
Only 1% of the Protein Data Bank (PDB) entries contain RNA alone. Multiple sequence alignments (used in AlphaFold) can fall short in human targets and engineered sequences. Therefore, RNA structure and function prediction underdeliver when compared to the protein prediction methods.
The team at AtomicAI used chemical mapping to generate an informative dataset to build ATOM-1, an RNA foundation model. Chemical mapping consists of modifying RNA nucleotides in a way that, once sequenced, can indicate the structure of the RNA molecule.
Using probe networks, which can take embeddings from ATOM-1 as input, the authors demonstrated that the model outperforms popular models when predicting tertiary RNA structures and is highly accurate when predicting secondary RNA structures.
Combinatorial prediction of therapeutic perturbations using causally-inspired neural networks [Gonzalez et al., bioRxiv, January 2024]
Why it Matters: This study provides a more efficient and direct method for identifying therapeutic perturbations. The ability of PDGRAPHER to predict specific sets of therapeutic targets directly has the potential to revolutionize phenotype-driven drug discovery. It not only speeds up the process but also improves the accuracy of identifying potential therapeutic agents, potentially leading to more effective and personalized treatments.
The study introduces PDGRAPHER, a novel graph neural network model for identifying therapeutic perturbations. Unlike traditional methods that predict phenotype changes due to perturbations, PDGRAPHER directly predicts perturbagens – sets of therapeutic targets – required for achieving specific responses in disease treatment. This approach significantly improves the efficiency of phenotype-driven drug discovery, offering direct predictions of therapeutic targets and reducing computational load. The model's effectiveness was demonstrated across eight datasets of genetic and chemical perturbations, successfully predicting effective perturbagens and ranking therapeutic targets more accurately than existing methods.
ADMET-AI: A machine learning ADMET platform for evaluation of large-scale chemical libraries [Swanson et al., BioRxiv, December 2023]
Why It Matters: ADMET-AI is able to quickly and accurately predict ADMET properties of large-scale chemical libraries – a crucial task for identifying potential drug candidates. This tool could significantly reduce the time and cost of drug discovery, allowing for a more efficient progression of compounds from discovery to clinical trials.
The study introduces ADMET-AI, a platform that utilizes a graph neural network, Chemprop-RDKit, for rapid and accurate ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity) predictions in drug discovery. The model significantly outperforms existing tools in speed and accuracy. ADMET-AI is available both as a web interface and a Python package for local prediction. It ranks highest on the TDC ADMET Benchmark Group leaderboard and is the fastest ADMET predictor, offering contextualized predictions using a reference set of approved drugs.
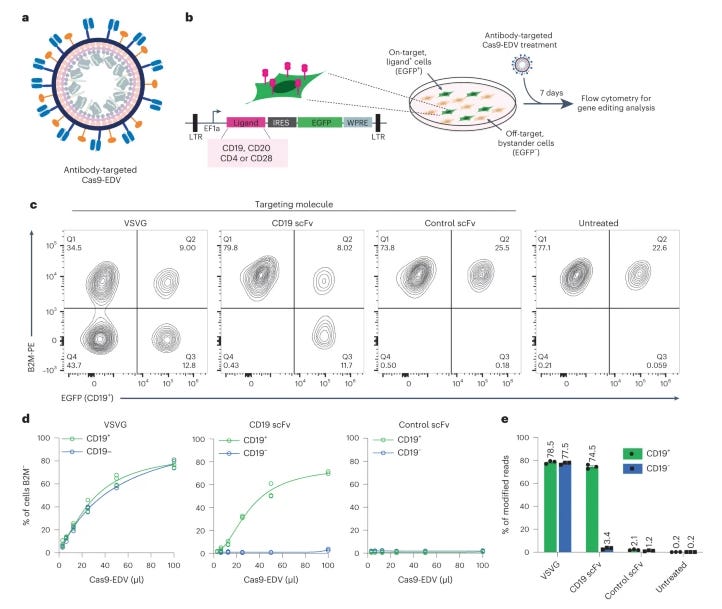
In vivo human T cell engineering with enveloped delivery vehicles [Hamilton et al., Nature Biotechnology, 2024]
Why it matters: Cell engineering has been bottlenecked by limitations in cell-type delivery. In this paper, Hamilton et al develop Cas9-packaging enveloped delivery vehicles (Cas9-EDVs) that have antibody fragments displayed on the surface, enabling transient delivery of CRISPR machinery to target cells, even in mixed populations in vivo. This work unveils a novel, programmable delivery method that shows promise in the therapeutic arsenal for enabling cell-type specific engineering.
Classic delivery approaches, such as lipid nanoparticles or virus vectors, are largely limited to the liver in vivo. Although recent advances have expanded the range of delivery to extrahepatic organs, there will be a need for multiple approaches to enable specificity. Here, the authors take advantage of the vesicular stomatitis virus glycoprotein (VSVG), which enables endosomal fusion but lacks native LDL receptor binding (a key liver receptor), and pair it with antibody-derived single-chain variable fragments (scFvs) on EDVs. By using retroviral viral-like particle assembly, which package Cas9 RNP inside, the authors demonstrate the ability to enable in vivo CAR-T cells in mice, with no liver delivery. This paper reveals the potential of EDVs for enabling therapeutic delivery, and opens the aperture for further exploration of fusion-based cargo delivery.
Notable Deals
Biotech Weather Report
Investors and executives across biopharma appear more upbeat about the industry and markets heading into 2024 as compared with 2023. A steady flow of clinical-stage M&A, IPOs and potential interest rate cuts on the horizon are contributing to positive sentiment.
M&A
Big pharma is focused on late-stage clinical and commercial assets to fill near-term revenue gaps and capitalise on big trends (ADCs, neuropsych, obesity, RLTs).
BMS / Karuna: $14B acquisition at 53% premium, neuropsych
J&J / Ambryx: $2B at 105% premium to last close, ADC development using synthetic amino acids
BMS / Rayze Bio: $4.1B at 104% premium to last close, radioligand therapy
Filed for IPOs
Five biotechs have filed for IPOs this month
Alto Neuroscience - mid-stage, CNS
CG oncology - late stage, oncology
ArriVent - late stage, oncology
Metagenomi - preclinical stage, gene editing
Kyverna - clinical stage, CAR-T for autoimmune
Financings
Tr1x: $75M launch to develop a novel class of regulatory T cell-based products to cure autoimmune and inflammatory diseases.
Disco: €20M launch for surface proteomics technology for target discovery, backers include AbbVie and Sofinnova.
ZymoChem: $21M Series A for biomanufacturing everyday products with increased yields.
Partnerships
Stealthy Isomorphic has inked two high value discovery partnerships on difficult-to-drug targets.
Novartis: $37.5M upfront, $1.2B milestones (3 targets)
Lilly: $45M upfront, $1.7B milestones (‘several’ targets)
Recently launched with $213M, the ‘fourth generation’ gene editing biotech has a healthy BD strategy to acquire complementary technologies:
Replace (acquisition): $65M upfront / near-term milestones, $185M total. Brings on an entirely new method of making small edits, adding to Tome’s PGI toolset currently focused on creating large insertions.
Genevant Sciences (partnership): $114M total deal size, already a well-invented lipid nanoparticle technology that makes sense to acquire through BD rather than internal R&D.
Novo Nordisk and Flagship Pioneering
The Danish pharma has created two separate partnerships with two Flagship portfolio companies under their broad framework collaboration. The aim for Novo is to expand its R&D in obesity with novel biology and chemistry.
Cellarity: aim to utilise high dimensional, transcriptomic data to design medicines against MASH
Omega Therapeutics: aim to develop a programmable epigenetic controller targeting thermogenesis rather than traditional appetite regulation approaches such as GLP-1s
MOMA: $66M upfront to develop 6 new precision oncology programs
Remix: $30M upfront to develop RNA processing modulators
MediLink: $50M upfront in collaboration on a c-Met-targeted ADC
Flagship’s ‘enabling technologies’
The moderna-creator has launched a company-wide initiative in ‘enabling technologies’ striking partnerships with technology giants to help create novel hardware and tools for Flagship’s portfolio companies as well as form the basis of new ventures. Flagship has partnered with Samsung and Thermofisher for this initiative.
What we listened to
In case you missed it
The Race to Put Brain Implants in People is Heating Up - Wired
2023: A Review of the Year in Neuroscience - The Spike, Medium
Alphabet's AI Unit Isomorphic Inks Drug Discovery Deals with Eli Lilly, Novartis for up to $3B - Endpoints News
Breakthrough in Functional Annotation with HiFi NN - NVIDIA Developer Blog
What we liked on socials channels
Field Trip
Did we miss anything? Would you like to contribute to Decoding Bio by writing a guest post? Drop us a note here or chat with us on Twitter: @ameekapadia @ketanyerneni @morgancheatham @pablolubroth @patricksmalone