BioByte 096: brain shuttles for antibody delivery, immune privilege in the brain, DNA data storage, clinical translation of spatial biology
Welcome to Decoding Bio’s BioByte: each week our writing collective highlight notable news—from the latest scientific papers to the latest funding rounds—and everything in between. All in one place.
Happy Friday fam! Quick housekeeping - If you’re an entrepreneurial scientist interested in starting a neuro company, consider applying to the Future Neuro Founders workshop hosted by PsyMed and KdT Ventures.
What we read
Blogs
The Dawn of Spatial Medicine [Eric Topol, Ground Truths, Oct 2024]
Spatial transcriptomics was named ‘Method of the Year’ in 2020 by Nature Methods. Spatial biology then rapidly expanded to spatial multi-omics, bringing biologic data with imaging to provide spatial resolution.
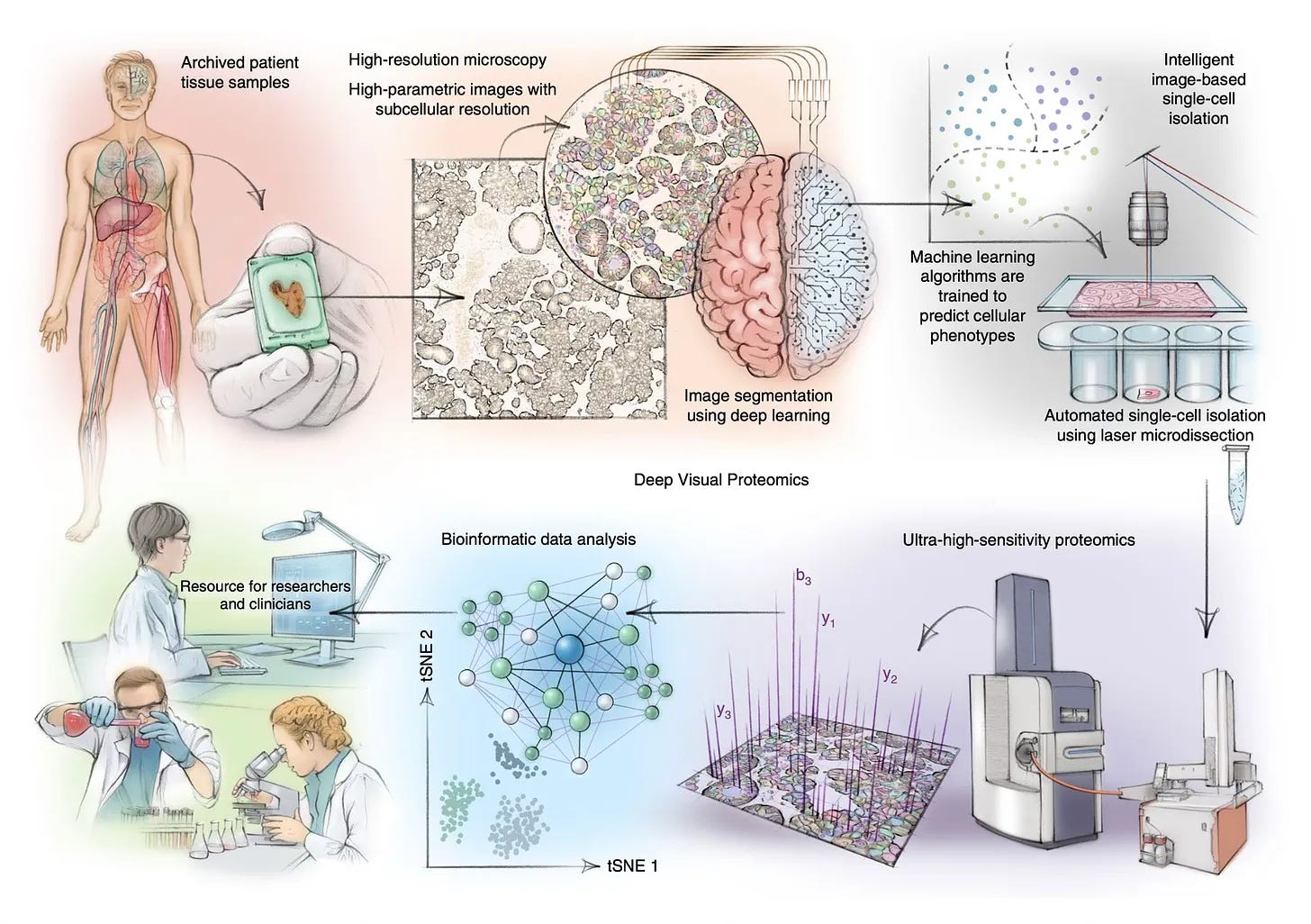
In 2022, Mathias Mann et al., developed Deep Visual Proteomics. In this method, microscopy and deep learning are combined to image a patient tissue sample, which identifies the cells to be analyzed. The laser miscrodissection then excises single cells which are then analyzed using mass spec-proteomics. In a new paper, the same method is used to investigate a new condition called toxic epidermal necrolysis (TEN), a fatal drug-induced condition. In the paper, patient samples with three different skin conditions were processed. The analysis showed that TEN patients specifically had marked increased cytokine activation which invoked the JAK/STAT pathway. Only the cell-type resolved proteomics allowed to uncover this result, as bulk proteomics principal components all overlapped across the three conditions.
The authors went on to test JAK inhibitors in cell culture and mouse models. They then treated seven patients with the JAK inhibitor. All patients fully resolved with no side effects. As Topol writes: this marks the first time we’ve seen spatial omics directly used in medicine to influence patient care.
Pest control gets the CRISPR treatment [Caroline Seydel, Nature Biotechnology, Oct 2024]
In June 2024, the company Agragene released genetically modified fruit flies on berry farms in California and Oregon, moving the precision-guided sterile insect technique (pgSIT) out of the lab and onto the field.
The spotted wing drosophila is the number one problem for many berry growers, as it is resistant to many chemical pesticides and fruit growers suffer large economic losses due to the pest.
SIT has been used since the 50s, but the traditional method is laborious and unspecific: the insects have to be separated by sex and then sterilized using radiation. Agregene, on the other hand, streamlines both processes. They breed two separate lines of fruit flies, one that carries the gene encoding Cas9 and the other sequences for guide RNAs. One of the targeted genes is necessary for female survival and the other for male fertility. When the two lines are mated, all the surviving eggs develop into sterile males.
The company is now collecting data from its field trials to prepare applications for the regulatory agencies. In contrast to gene drive methods, the flies do not transfer genetic material and do not proliferate themselves.
Tumor atlases enable researchers to navigate through cancers [Singer et al., Nature Research Briefing, Oct 2024]
The Human Tumor Atlas Network's (HTAN) is an initiative under the U.S. Cancer Moonshot to create multidimensional atlases of tumors across various cancer types and shapes. These atlases, encompassing 21 tissue sites from nearly 2,000 individuals, leverage advanced techniques like single-cell RNA profiling and 3D imaging to map the molecular and cellular interactions within tumors. This research aims to foster collaborative data sharing and is available to the public. Future efforts will focus on expanding the tumor atlas data, improving predictive models for clinical use, and validating these in clinical studies to optimize cancer care. Some key findings:
Collective cell behavior: Contrary to the belief that single cells drive colorectal cancer, HTAN research shows that multiple cells often collectively seed these tumors.
Tumor plasticity: Findings reveal that the metastatic microenvironment can promote plasticity, aiding tumors in resisting treatment and enabling them to undergo lineage reprogramming.
Immune interactions: By visualizing tumor-immune cell interactions in 3D, the research provides insights into how immune cells interact within the tumor microenvironment, impacting tumor behavior and progression.
‘Do-it-yourself’ data storage on DNA paves way to simple archiving system [Carina Imburgia & Jeff Nivala, Nature News and Views, October 2024]
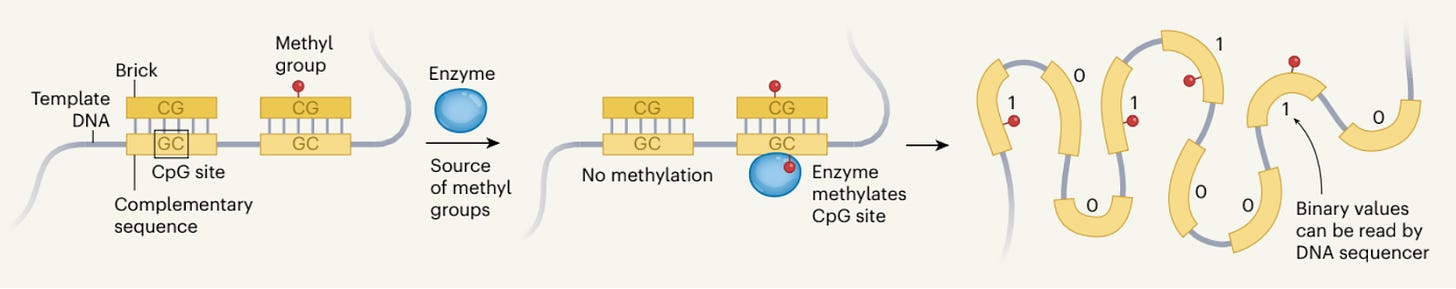
Since the onset of the Information Age with the invention of the transistor in the mid 20th century, the amount of data generated annually has grown dramatically. More than 100 zettabytes of data—one zettabyte being 1021 bytes or about 250 quadrillion photos worth of data—have been generated in the past year alone. This ever-increasing data supply will soon render traditional data storage methods obsolete. DNA-based data storage serves to be a promising alternative due to its inherent stability and significantly increased storage density, as one gram of DNA can hold up to 215,000 terabytes of data. However, to store data with DNA currently, the individual DNA strands must be synthesized one nucleotide at a time, a very time-consuming, expensive, and mistake-prone process. Zhang et al. propose an alternative method of data storage on DNA that is faster and more robust using what they call epigenetic bits, or epi-bits.
The novel manner in which Zhang et al. encode data on DNA begins with a predetermined template sequence of DNA. This sequence contains within it a series of unique binding regions, each of which hosts a CpG site. To imprint data onto the template, a DNA “brick” corresponding to one of the unique binding regions is added to the template, containing either a methylated or unmethylated CpG group. Once the brick binds to the complementary sequence, an enzyme can methylate the CpG site on the template, modifying it to contain the data. The template is then read out with great accuracy using an inexpensive, portable DNA reader, where methylated sites are interpreted as binary ones and unmethylated sites read as binary zeros.
The authors also demonstrate the ability of this technology to scale. The length of the template is practically limited to about 1300 nucleotides, and there is a tough balance between the uniqueness of the DNA brick and the length of the brick that confine the number of bits per template. To circumvent this limitation, DNA barcodes were used to distinguish between otherwise identical templates, effectively allowing the authors to break down larger files into a set of smaller ones and read them with very few errors. To demonstrate the robustness of this method, they also sourced 60 volunteers from various academic backgrounds to encode some data, receiving only a 1.4% error rate in the bits encoded.
Despite the benefits of this technique, including its robustness, compatibility with automation, and significantly increased bit-parallelism (number of bits added simultaneously) relative to single nucleotide DNA synthesis, it still has some significant limitations. It is still more expensive than traditional single-nucleotide DNA synthesis, and the methylated sites are not copied during DNA replication techniques like PCR. The stability of the methylated sites also remains unknown. Additionally, in order to access any of the data, the entire data set would need to be sequenced, which is a substantial time burden. Nevertheless, if these limitations can be addressed, there is significant potential for this pioneering process to revolutionize DNA-based data storage.
Papers
Enhanced delivery of antibodies across the blood-brain barrier via TEMs with inherent receptor-mediated phagocytosis [Edavettal et al., Med, December 2022]
We’re resurfacing a paper from a couple years ago to highlight the technology involved in the $1.4B acquisition of Aliada Therapeutics by AbbVie. Aliada is developing a novel technology for engineering antibodies to cross the blood-brain barrier (BBB), a key bottleneck for developing therapeutic antibodies for CNS diseases.
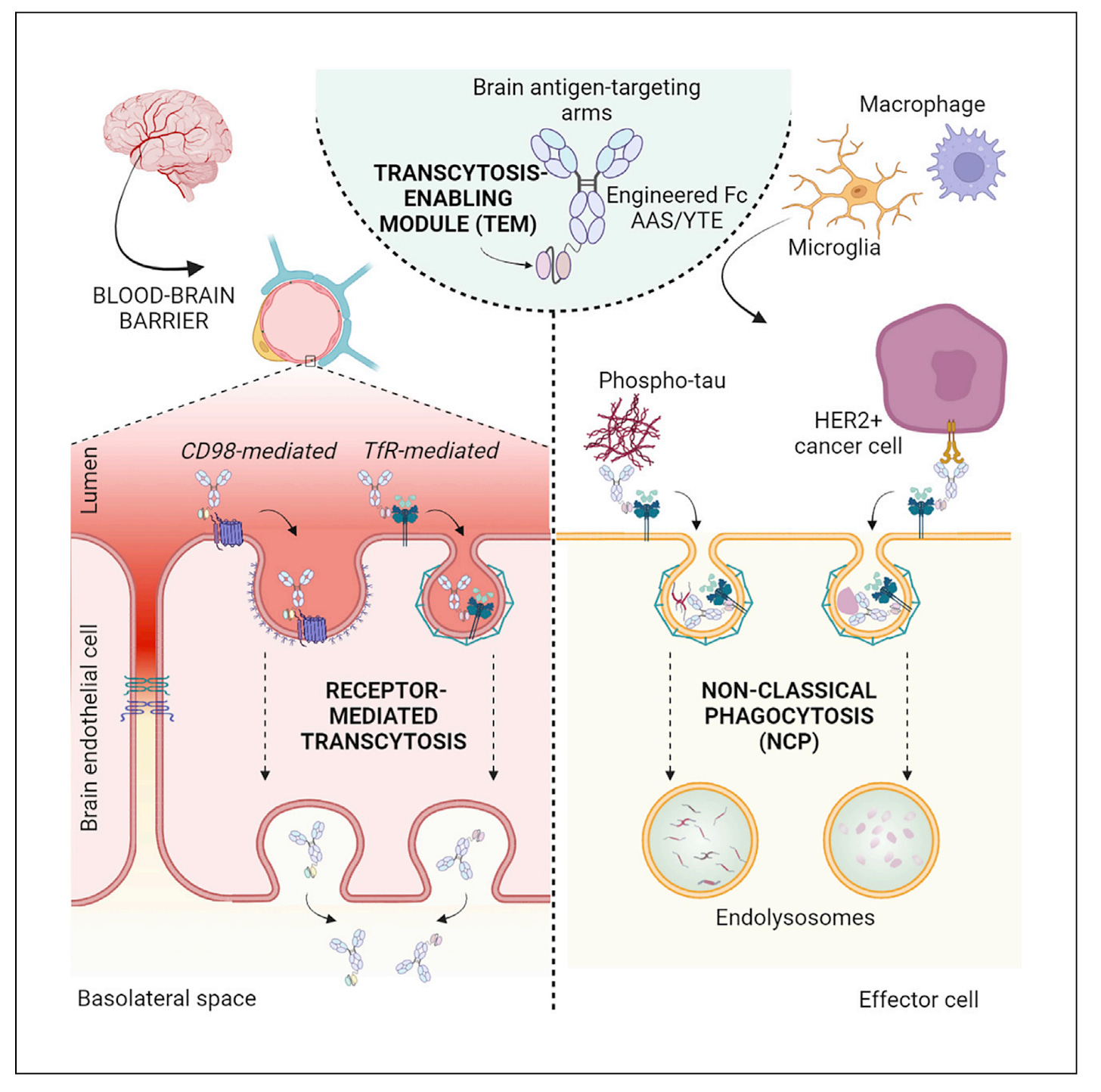
Researchers at J&J (where Aliada was spun out) developed a new antibody delivery system called transcytosis-enabling modules (TEMs) to improve transport across the BBB. Getting therapeutic antibodies into the brain has been a persistent challenge, with typical antibodies achieving only 0.1-0.2% penetration across the BBB.
The TEM platform combines optimized binding modules that transport across the BBB via transferrin receptor (TfR) or CD98, along with an engineered antibody design that prevents unwanted side effects like reticulocyte depletion. A key innovation is the platform's novel non-classical phagocytosis (NCP) mechanism, which clears disease targets with reduced inflammatory response compared to conventional antibodies. Testing in non-human primates showed up to 22-fold increased brain exposure compared to standard antibodies, with effective clearance of both tau protein aggregates and cancer cells.
Aliada is currently running a PhI clinical trial using the TEM technology for the delivery of an anti-amyloid antibody for Alzheimer’s. By leveraging the brain shuttle technology, the hope is that lower doses of drug can be used while maintaining similar concentrations of antibody in the brain, driving down side effects and maintaining efficacy.
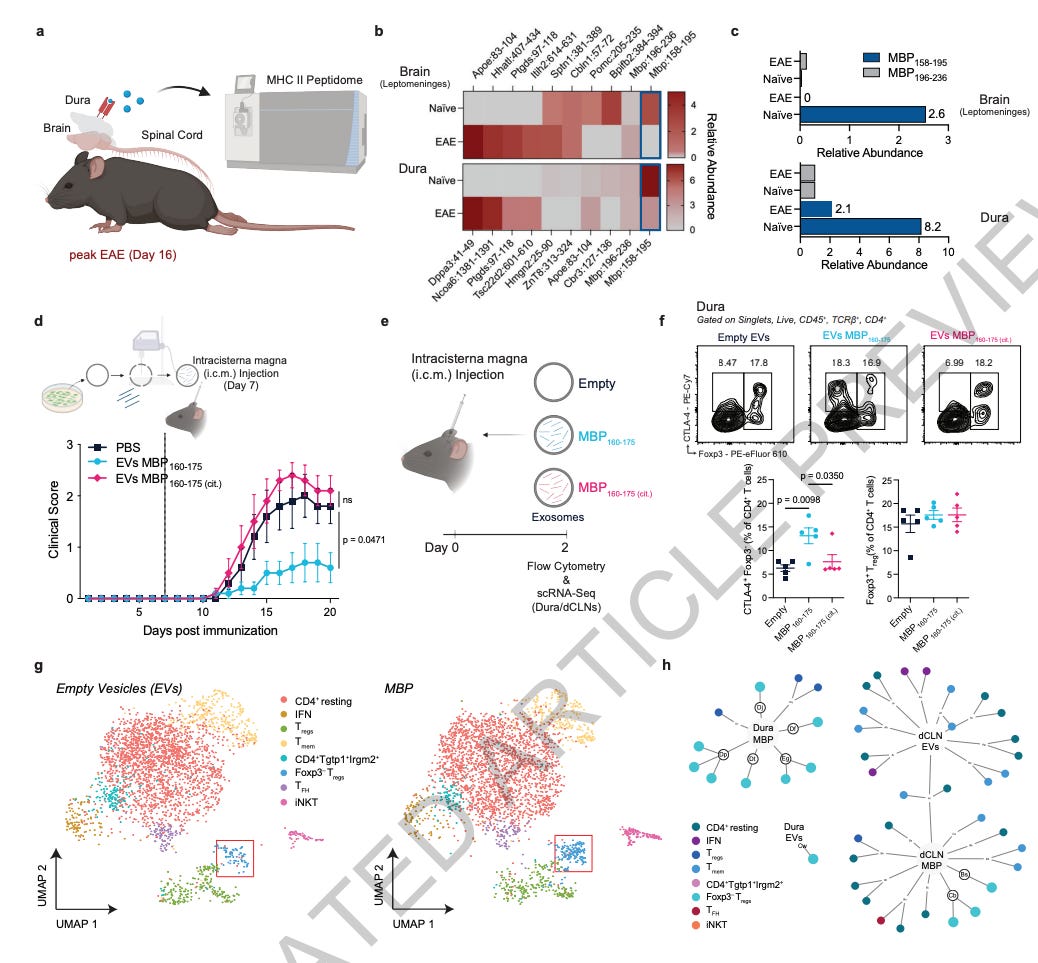
Endogenous self-peptides guard immune privilege of the central nervous system [Kim et al., Nature, 2024]
Work over the past decade has challenged the traditional view of central nervous system immune privilege as merely a physical barrier system (via the blood brain barrier), and has demonstrated the presence of active cross-talk between the CNS and immune system. In this paper, the authors discovered that the CNS actively maintains its immune privilege status through the presentation of specific self-peptides – particularly those derived from myelin basic protein – on MHC II molecules at the brain’s borders and draining lymph nodes.
The authors used a number of techniques to interrogate the dynamics of immune cross-talk in mice, such as mass spectrometry to analyze MHC II-bound peptides, scRNA sequencing to characterize immune populations, MHC II tetramers to track specific T-cells, and flow cytometry to identify specific cell subsets. After screening peptides for their encephalitogenicity, they delivered them to the CSF via extracellular vesicles to test their efficacy as a potential antigen-specific immunotherapy.
A key discovery was that during neuroinflammation, the presentation of these regulatory self-peptides (particularly MBP158-195) significantly decreased. The researchers found that different types of antigen-presenting cells varied in their ability to present these peptides, with B cells showing limited capacity compared to myeloid cells. Importantly, when they supplemented these regulatory peptides through targeted delivery to the CSF, they observed significant suppression of autoimmune responses through the expansion of suppressor CD4+ T cells that operated via CTLA-4 and TGF-β mechanisms.
This work has significant therapeutic implications for neuroinflammatory and autoimmune diseases, such as multiple sclerosis. The presence of these guardian peptides expands the armament of antigen-specific immunotherapy approaches. Delivering such regulatory peptides to the CNS could avoid broad immunosuppression associated with current treatments.
Notable deals
David Baker’s Archon Biosciences raises $20M seed for a novel antibody-based therapeutic.The biotech is developing “antibody cages,” which combine a generative AI-designed protein with a standard antibody to enhance it with novel properties. These new antibody structures have the potential to improve biodistribution and increase target engagement.
Novo Holdings leads Kivu Bioscience’s $92M Series A to support next generation ADCs. Kivu’s aim is to improve on existing pain points of ADCs including increased stability and more precise targeting with hopes to create best-in-class assets in this hot market. Kivu is licensing Synaffix’s site-specific linker-payload technology to window the therapeutic window.
Iambic Therapeutics has introduced Enchant, an AI model that takes trains on multiple data modalities (both preclinical and clinical) and is designed to predict the success of molecules in their earliest stages. Enchant achieves predictive accuracy in forecasting pharmacokinetic properties like drug half-life. Iambic released a brief white paper on the model which has a few examples show-casing the model’s potential.
Novartis licenses Monte Rosa’s VAV1 molecular glue for $150M upfront. The asset is currently undergoing Phase 1 studies for immune-mediated conditions and Novartis will obtain exclusive rights for development and commercialisation. Monte Rosa is entitled to up to $2.1B in milestones if all met, however royalties remain limited to ex-US sales.
What we liked on socials channels
Events
Field Trip
Did we miss anything? Would you like to contribute to Decoding Bio by writing a guest post? Drop us a note here or chat with us on Twitter: @ameekapadia @ketanyerneni @morgancheatham @pablolubroth @patricksmalone